Periodic Table Practice Worksheet Answers
Are you a student looking to improve your understanding of the periodic table? Look no further! In this blog post, we will provide you with the answers to a periodic table practice worksheet. By having access to these answers, you can effectively review and reinforce your knowledge of the elements and their properties.
Table of Images 👆
- Periodic Table Puns Worksheet Answers
- Naming Ionic Compounds Worksheet Answers
- Covering and Surrounding Math Book Answer Key
- Protons Neutrons and Electrons Worksheet
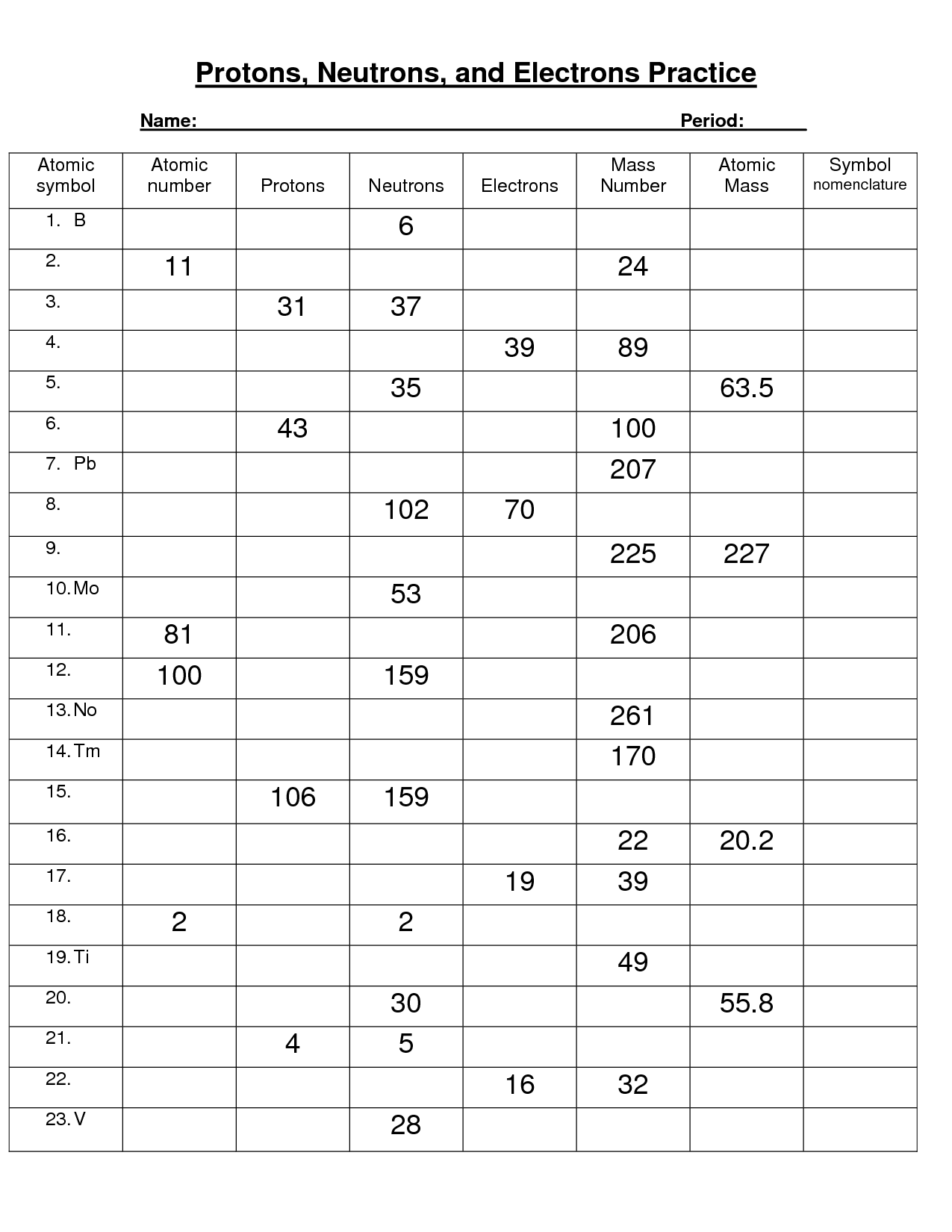
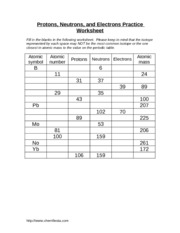
- Protons Neutrons Electrons Practice Worksheet Answers
- Alien Periodic Table Answer Key
- Worksheets Answer Key
- Periodic Table Activity Worksheets
- Isotope Practice Worksheet Answers
- Atoms and Molecules in Chemical Formulas Worksheet
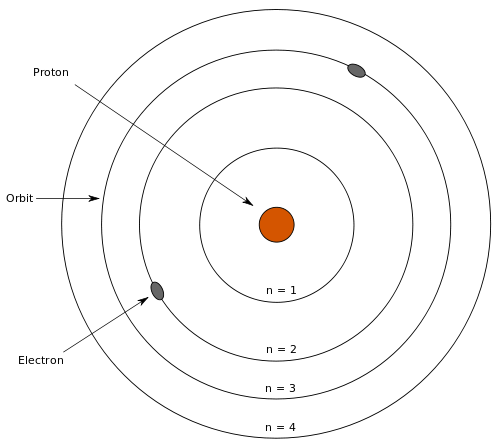
- Bohr Model Diagram
- Bohr Model Diagram
- Bohr Model Diagram
- Bohr Model Diagram
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the periodic table?
The periodic table is a tabular arrangement of chemical elements ordered by their atomic number, electron configuration, and recurring chemical properties. It provides a systematic way to organize and visualize the relationships between elements based on their characteristics, such as atomic structure and reactivity, which helps scientists predict element behavior and understand the underlying principles of chemistry.
How many elements are there in the periodic table?
There are 118 elements in the periodic table.
What information does each element's square on the periodic table provide?
Each element's square on the periodic table provides information such as the element's atomic number, chemical symbol, atomic mass, and sometimes its name. It also often includes other important details such as the element's electron configuration, melting and boiling points, density, and other key properties. The periodic table is a valuable tool for organizing and understanding the characteristics and relationships between different elements.
What is the atomic number of an element?
The atomic number of an element is the number of protons found in the nucleus of an atom of that element. It is a unique identifier for each element on the periodic table and determines the chemical properties of that element.
How is the periodic table organized?
The periodic table is organized by arranging elements in order of increasing atomic number from left to right and top to bottom. Elements in the same column, called groups, have similar chemical properties due to their similar electron configurations. The rows, called periods, represent the number of electron shells in an atom. This layout allows us to easily determine the characteristics and behaviors of elements based on their placement in the table.
What are periods in the periodic table?
Periods in the periodic table are horizontal rows that categorize elements based on the number of electron shells they have. Each period represents the number of electron shells an element's atoms possess, with elements in the same period having the same number of electron shells. There are a total of 7 periods in the periodic table, each indicating the energy levels and electron configurations of the elements within it.
What are groups or families in the periodic table?
Groups, also known as families, in the periodic table are columns of elements with similar chemical properties due to having the same number of electrons in their outermost shell. There are 18 groups in the periodic table, each denoted by a number or letter, such as Group 1 (alkali metals) or Group 17 (halogens). Elements within the same group tend to react in similar ways and share common characteristics.
What are the properties of metals, nonmetals, and metalloids?
Metals are typically shiny, malleable, ductile, and good conductors of heat and electricity. Nonmetals are usually dull, brittle, poor conductors of heat and electricity, and often gain electrons in chemical reactions. Metalloids have properties that are intermediate between metals and nonmetals, such as being semiconductors of electricity and having varying degrees of malleability and conductivity.
Why are elements grouped together in the periodic table?
Elements are grouped together in the periodic table based on their similar chemical properties. By organizing elements in groups, it becomes easier to predict the properties and behaviors of elements within a group, as they tend to share similar characteristics. This grouping is due to the elements having the same number of valence electrons, which influences their reactivity and bonding patterns.
How can the periodic table be used to predict the properties of elements?
The periodic table can be used to predict the properties of elements based on their position within the table. Elements that are in the same group or column tend to have similar properties, such as reactivity and chemical behavior, due to their similar electronic configurations. In addition, elements from left to right across a period show a gradual change in their properties, with metals on the left and nonmetals on the right. By understanding the trends in the periodic table, such as atomic size, electronegativity, and ionization energy, scientists can make informed predictions about the properties of elements.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments