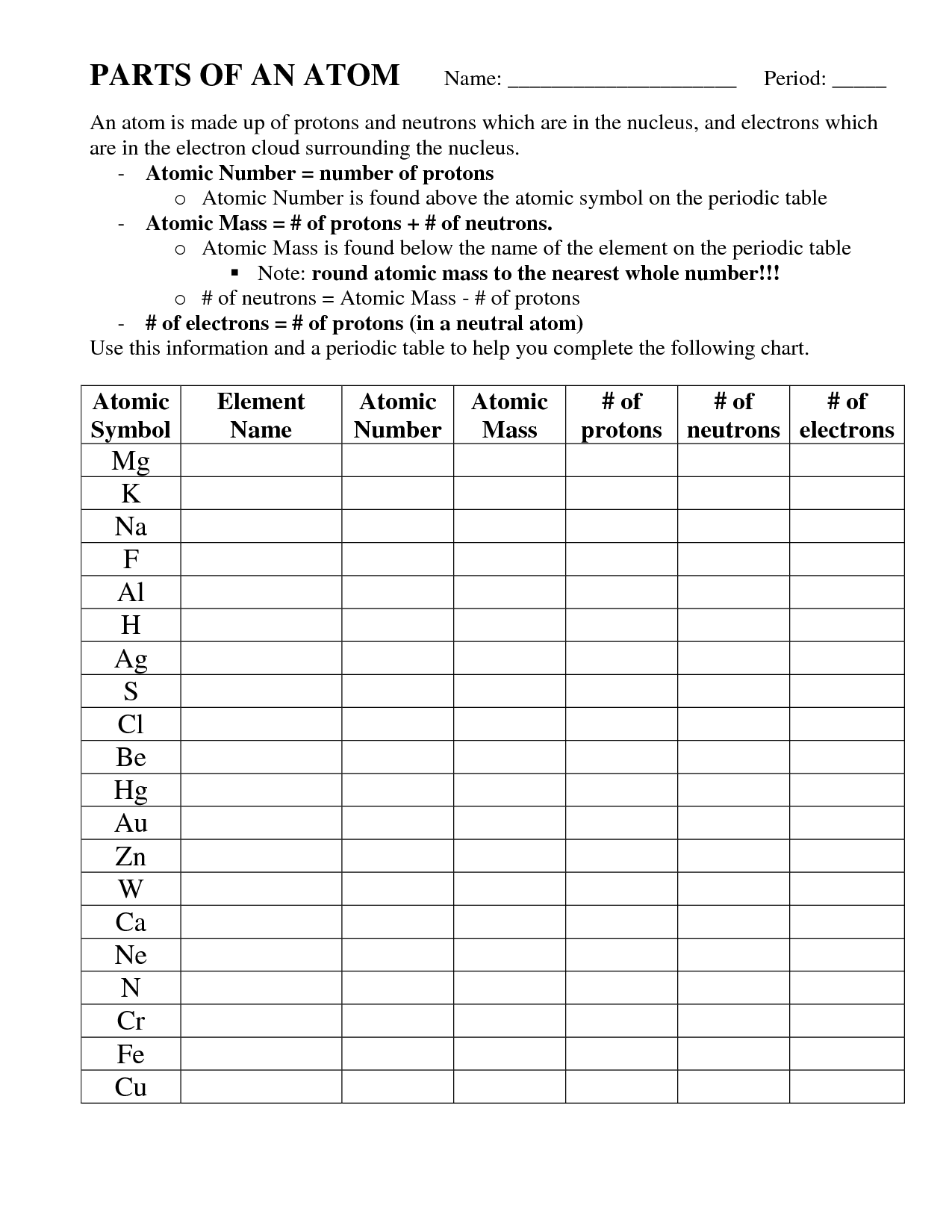
Parts of an Atom Worksheet
Are you a student or educator in need of a helpful resource to reinforce your understanding of the various parts of an atom? If so, you're in luck! In this blog post, we will explore the benefits of utilizing worksheets as an effective tool for learning and practicing the concept of atomic structure.
Table of Images 👆
- Electrons in Atoms Worksheet Answers
- 6th-Grade Atom Parts Worksheet
- History of Atom Models Worksheet
- ESL Body Parts Worksheet
- ESL Body Parts Worksheet
- Insect Parts Worksheets for Kids
- Half Circle Outline
- English Food Worksheets for Kids
- Atoms vs Ions Worksheet Answers
- Train Outline Drawing
- Kindergarten Missing Letter Worksheets
- Rooms in My House Worksheet
- Tree Branches Silhouette
- Microscope Parts Blank Diagram
- Printable Face Parts for Kids
- Worksheet On Comma Splices and Run
- Worksheet On Comma Splices and Run
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is the nucleus of an atom?
The nucleus of an atom is the central core that contains protons and neutrons, which are tightly bound together by strong nuclear forces. It contributes to most of the atom's mass and has a positive electric charge due to the presence of protons. The nucleus is surrounded by a cloud of electrons that orbit around it in specific energy levels.
What are protons?
Protons are subatomic particles that have a positive electrical charge and are found within the nucleus of an atom. They are crucial in determining the element's identity and are involved in chemical reactions and the creation of different elements through nuclear fusion and fission processes.
What are neutrons?
Neutrons are subatomic particles found in the nucleus of atoms, alongside protons. They have no electrical charge, and their main function is to add mass and help stabilize the nucleus of the atom. Neutrons play a crucial role in nuclear reactions and are essential for the existence of all matter in the universe.
What is the atomic number?
The atomic number is the number of protons in the nucleus of an atom, which also determines the element's identity on the periodic table.
What is the mass number?
The mass number is the total number of protons and neutrons in the nucleus of an atom. It is used to identify different isotopes of an element and is usually written as a superscript before the chemical symbol of the element.
What are electrons?
Electrons are subatomic particles with a negative charge that orbit the nucleus of an atom in specific energy levels. They play a fundamental role in chemical bonding, electricity, and many other physical processes.
What is the electron cloud?
The electron cloud refers to the region around an atomic nucleus where electrons are most likely to be found. It represents the probability distribution of finding an electron in a particular area around the nucleus. The electron cloud is a way to visualize the distribution of electrons in an atom, showing that electrons do not follow fixed paths but instead exist in a cloud-like structure where their exact location cannot be precisely determined.
What is an energy level or electron shell?
An energy level, also known as an electron shell, is a region around the nucleus of an atom where electrons are most likely to be found. Electrons occupy these shells based on their energy levels, with the innermost shell having the lowest energy level and subsequent shells increasing in energy. Each shell can hold a specific number of electrons, with the outermost shell determining an atom's chemical properties.
What is the valence shell?
The valence shell is the outermost shell of an atom, which contains the electrons involved in forming chemical bonds with other atoms. The number of electrons in the valence shell determines an atom's reactivity and its ability to participate in chemical reactions.
What is the atomic mass unit (amu)?
The atomic mass unit (amu) is a unit of mass equal to 1/12 of the mass of one atom of carbon-12, which is approximately 1.66 x 10^-27 kilograms. It is commonly used to express the relative masses of atoms and molecules in chemistry and physics.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments