Osmosis Worksheet Answer Key Biology
Are you a biology student looking for a reliable and comprehensive resource to practice your understanding of osmosis? Look no further! In this blog post, we will be providing you with an answer key to an osmosis worksheet that covers the essential concepts and questions related to this fascinating process. Whether you are a high school student studying for an upcoming exam or a college student looking to strengthen your knowledge, this answer key is perfect for you. Let's dive in and explore the world of osmosis together!
Table of Images 👆
- Cell Structure and Function Worksheet Answers
- AP Biology Meiosis Worksheet Answer Key
- The Cell Cycle and Cancer Virtual Lab Worksheet Answers
- Diffusion and Osmosis Worksheet Answer Key
- Cell Membrane Coloring Worksheet Answers Chapter 7
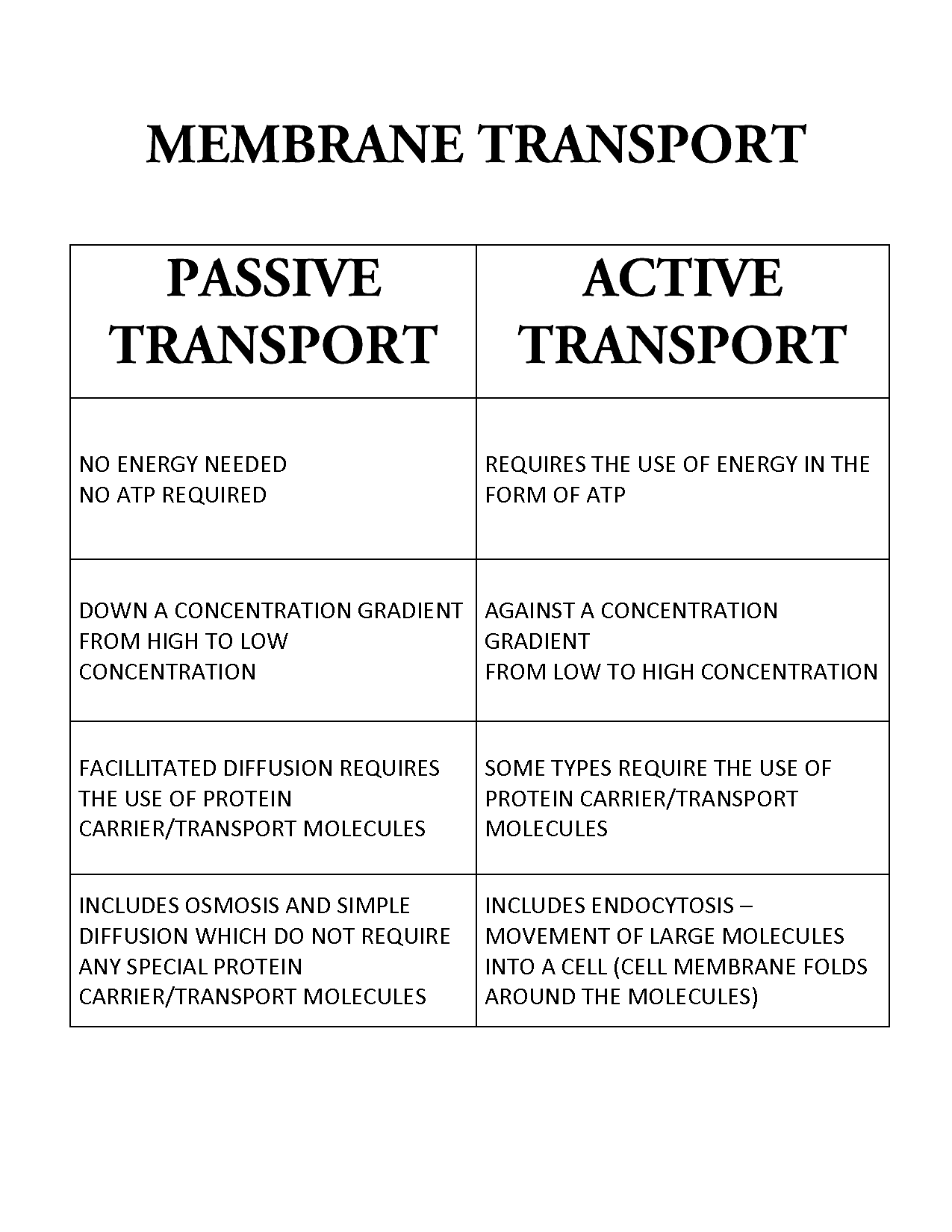
- Active vs Passive Transport Worksheet
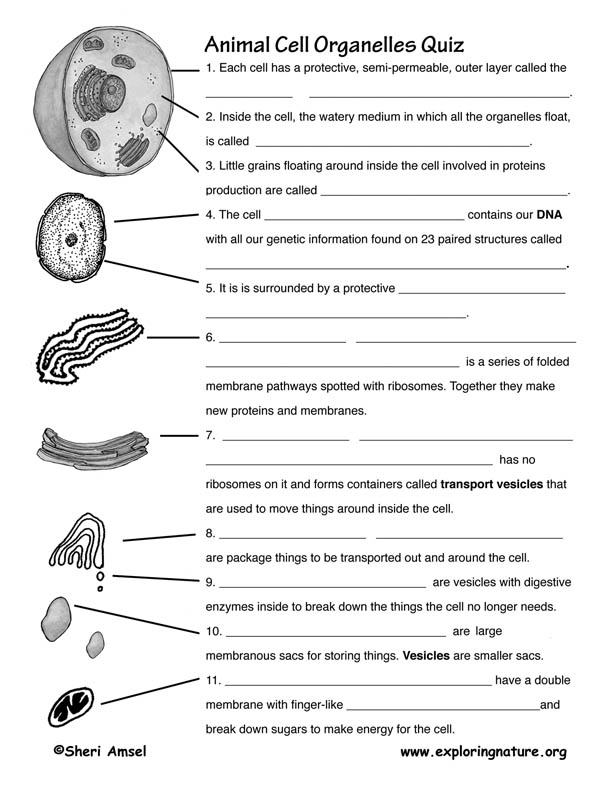
- Biology Cell Organelles Worksheet
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
- Transition Between Photosynthesis and Cellular Respiration
More Biology Worksheets
Free Printable Biology WorksheetsCollege Biology Worksheets
7th Grade Biology Worksheets
Biology Macromolecules Worksheets and Answers
Karyotype Worksheet Answers Biology
What is osmosis?
Osmosis is a process in which solvent molecules (usually water) move through a semipermeable membrane from an area of low solute concentration to an area of high solute concentration, in order to equalize the concentration of solutes on both sides of the membrane. This movement of water across the membrane allows for the balance of solute concentrations and plays a crucial role in various biological and chemical processes.
How does osmosis differ from diffusion?
Osmosis is a specific type of diffusion that involves the movement of water molecules across a selectively permeable membrane from an area of high water concentration to an area of low water concentration. Diffusion, on the other hand, refers to the overall movement of particles from an area of high concentration to an area of low concentration, regardless of the type of particle being transported. In summary, osmosis is a form of diffusion that specifically involves the movement of water molecules.
What are the three types of solutions in osmosis?
The three types of solutions in osmosis are isotonic, hypertonic, and hypotonic. In an isotonic solution, the concentration of solutes is the same inside and outside the cell, resulting in no net movement of water. A hypertonic solution has a higher concentration of solutes outside the cell, causing water to move out of the cell, leading to cell shrinkage. Conversely, a hypotonic solution has a lower solute concentration outside the cell, resulting in water moving into the cell, causing it to swell or potentially burst.
How does water move across a selectively permeable membrane during osmosis?
During osmosis, water moves across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This movement occurs to equalize the concentration of solutes on both sides of the membrane. The water molecules pass through the membrane via osmosis to dilute the area with higher solute concentration and increase the concentration in the area with lower solute concentration until equilibrium is reached.
Describe hypotonic solutions and their effects on cells.
Hypotonic solutions are solutions with a lower solute concentration compared to the inside of a cell. When a cell is exposed to a hypotonic solution, water flows into the cell due to osmosis, leading to swelling and possibly bursting of the cell in a process called cytolysis. This is because the water moves from an area of higher concentration (the solution) to an area of lower concentration (inside the cell), causing the cell to expand as it takes in water.
Explain hypertonic solutions and their impact on cells.
Hypertonic solutions have a higher concentration of solutes compared to the solution inside the cells. When a cell is placed in a hypertonic solution, water molecules move out of the cell through osmosis, causing the cell to shrink and possibly undergo cellular dehydration. This can disrupt the normal cell functions, cause cell damage, and even lead to cell death if the exposure to the hypertonic solution is prolonged or severe.
What happens to cells in an isotonic solution?
In an isotonic solution, cells maintain their normal shape and size as the concentration of solutes inside and outside the cell is equal, resulting in no net movement of water across the cell membrane. This balance prevents the cell from either gaining or losing water, thus maintaining its equilibrium.
How does osmotic pressure influence the movement of water?
Osmotic pressure influences the movement of water by drawing water molecules from a hypotonic solution to a hypertonic solution across a semipermeable membrane. This movement of water helps to equalize the concentration of solute molecules on both sides of the membrane, ultimately balancing the osmotic pressure between the two solutions. This process continues until an equilibrium is reached, with the net movement of water dependent on the concentration gradient of solute particles and the osmotic pressure difference between the two solutions.
What role does the concentration gradient play in osmosis?
The concentration gradient plays a crucial role in osmosis as it is responsible for the movement of water across a semi-permeable membrane. Water molecules move from an area of higher water concentration to an area of lower water concentration in order to equalize the solute concentrations on both sides of the membrane. This movement of water through osmosis helps maintain the balance of water and solutes in cells and tissues, allowing for proper functioning and homeostasis within biological systems.
Can osmosis occur in living organisms and non-living systems alike?
Yes, osmosis can occur in both living organisms, such as cells in plants and animals, and non-living systems. Osmosis is the movement of solvent molecules from an area of lower solute concentration to an area of higher solute concentration through a semipermeable membrane. This process is important for maintaining proper balance and functioning in both living and non-living systems.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




















Comments