Organic Oxidation Reactions Worksheet
Are you a chemistry enthusiast looking to sharpen your understanding of organic oxidation reactions? Look no further than our Organic Oxidation Reactions Worksheet! This comprehensive worksheet is designed to help students grasp the concept of oxidation in organic chemistry by providing a variety of practice problems and explanations of key concepts. Whether you're a high school student preparing for an exam or a college student looking for additional practice, this worksheet is the perfect tool to enhance your understanding of this important subject.
Table of Images 👆
- Balancing Redox Reactions Worksheet Answers
- Balancing Redox Reactions Worksheet
- Balancing Redox Reactions Worksheet Answers
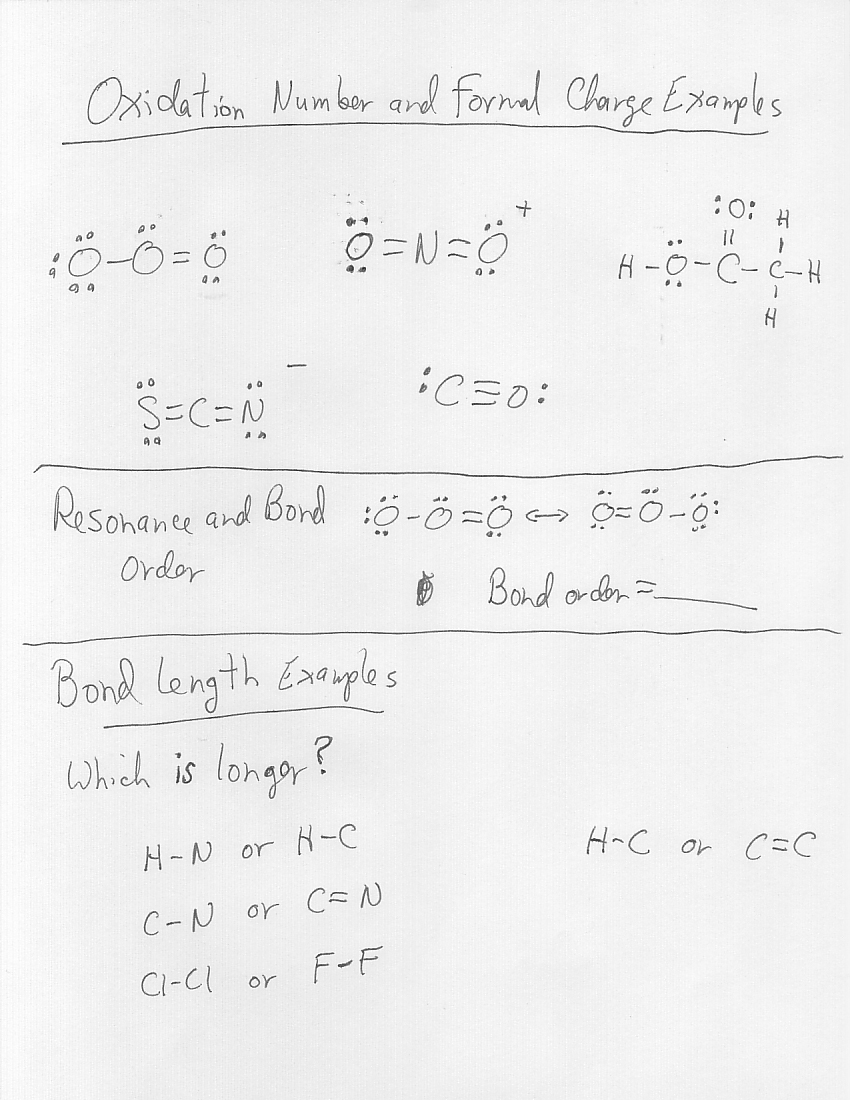
- Oxidation Number Rules Worksheet
- Organic Reactions Worksheet
- Types of Reactions Worksheet Answer Key
- Chemistry Single Replacement Reaction Worksheet
- Precipitation Reactions Worksheet
- Organic Chemistry Reactions Worksheet
- Balancing Chemical Equations Worksheet Answer Key
- Oxidation Numbers Worksheet
- Chemical Word Equations Worksheet Answers
- Balancing Redox Reactions Worksheet Answers
- Practice Balancing Chemical Equations Worksheet
- Organic Chemistry Naming Alkanes Worksheet
- Oxidation Numbers Worksheet Answers
- Functional Groups Organic Chemistry Cheat Sheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
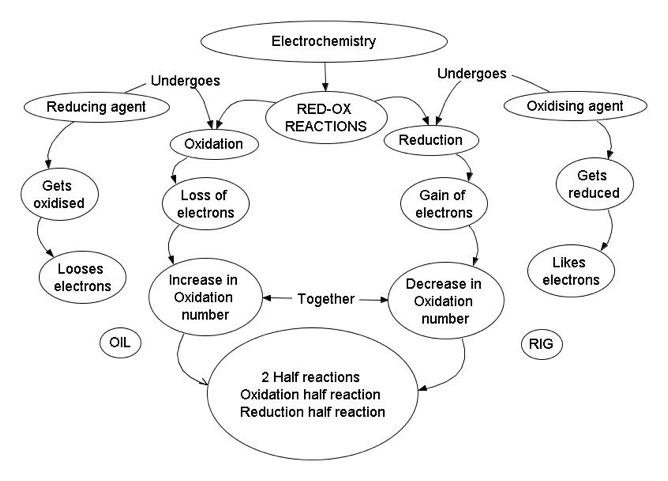
What are organic oxidation reactions?
Organic oxidation reactions involve the loss of electrons from a carbon-containing compound, resulting in an increase in the oxidation state of the carbon atom. These reactions often involve the transfer of electrons to an oxidizing agent, such as oxygen or a metal ion, leading to the formation of new bonds and functional groups on the organic molecule. Organic oxidation reactions are important in biological processes, such as metabolism, as well as in the synthesis of numerous organic compounds in the laboratory.
What are some common examples of organic oxidation reactions?
Some common examples of organic oxidation reactions include the conversion of primary alcohols to aldehydes and then to carboxylic acids, the oxidation of secondary alcohols to ketones, the oxidation of aldehydes to carboxylic acids, and the oxidation of alkyl side chains in aromatic compounds to form benzoic acids. Additionally, the oxidation of alkenes to form diols, and the conversion of alkyl groups to acyl groups through the use of oxidizing agents are also common organic oxidation reactions.
What is the purpose of organic oxidation reactions?
The purpose of organic oxidation reactions is to introduce oxygen atoms or remove hydrogen atoms from organic molecules, resulting in the formation of new functional groups and increased oxidation state. This process is essential for the synthesis of various compounds, including alcohols, ketones, carboxylic acids, and aldehydes, and plays a key role in the metabolism of living organisms by providing energy and facilitating biochemical transformations.
How are organic oxidation reactions different from other types of reactions?
Organic oxidation reactions involve the loss of electrons from a molecule, resulting in an increase in oxidation state. This is different from other types of reactions such as addition or substitution reactions, where the focus is on the addition of functional groups or the swapping of atoms. In organic oxidation reactions, the carbon atom in the molecule loses electrons, leading to the formation of new functional groups and potentially different chemical properties.
What is the role of oxidizing agents in organic oxidation reactions?
Oxidizing agents play a crucial role in organic oxidation reactions by accepting electrons from the organic substrate, thereby facilitating the conversion of lower oxidation states to higher oxidation states. This process leads to the generation of products with new functional groups and plays a key role in various chemical transformations, such as the conversion of alcohols to carbonyl compounds or the oxidation of alkenes to form diols. Oxidizing agents are essential in organic synthesis for introducing new functional groups and increasing the complexity of organic molecules.
What are some factors that can affect the rate of organic oxidation reactions?
Some factors that can affect the rate of organic oxidation reactions include the nature of the organic compound being oxidized, the type of oxidizing agent used, temperature, pressure, concentration of reactants, presence of catalysts, and the pH of the reaction environment. Additionally, steric hindrance, solvent polarity, and the presence of functional groups can also influence the rate of organic oxidation reactions.
Can organic oxidation reactions be reversed? Why or why not?
Organic oxidation reactions, by definition, involve the loss of electrons from the organic compound. This process cannot be directly reversed by simply adding electrons back as oxidation reactions involve complex rearrangements of bonds and functional groups. However, certain reactions or processes can be employed to reduce the oxidized organic compound back to its original form by using reducing agents or specific chemical reactions. These reactions are not a direct reversal of the oxidation process but can result in the reduction of the compound back to its original state.
How do organic oxidation reactions affect the functional groups in organic compounds?
Organic oxidation reactions can lead to the conversion of functional groups within organic compounds. For example, alcohols can be oxidized to form aldehydes or carboxylic acids, while aldehydes can be further oxidized to carboxylic acids. This change in functional groups can alter the chemical properties and reactivity of the organic compound, leading to the formation of new compounds with different properties.
Are organic oxidation reactions always exothermic?
Not necessarily. Organic oxidation reactions can be either exothermic or endothermic depending on the specific reaction and the reactants involved. While many oxidation reactions release energy in the form of heat (exothermic), there are also cases where energy is absorbed from the surroundings (endothermic) to drive the oxidation process. Overall, the energy change in organic oxidation reactions can vary depending on the specific reaction conditions and reactants.
What are some potential applications or significance of organic oxidation reactions?
Organic oxidation reactions have a wide range of applications and significance in various fields. They are crucial in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals, allowing for the introduction of functional groups and structural modifications. Organic oxidation reactions are also utilized in materials science for the production of polymers and in the development of new materials with specific properties. In addition, these reactions are important in biochemistry and environmental chemistry, playing a key role in metabolism, detoxification processes, and the degradation of organic pollutants. Overall, organic oxidation reactions are essential tools in organic chemistry with diverse applications in industry, research, and everyday life.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.

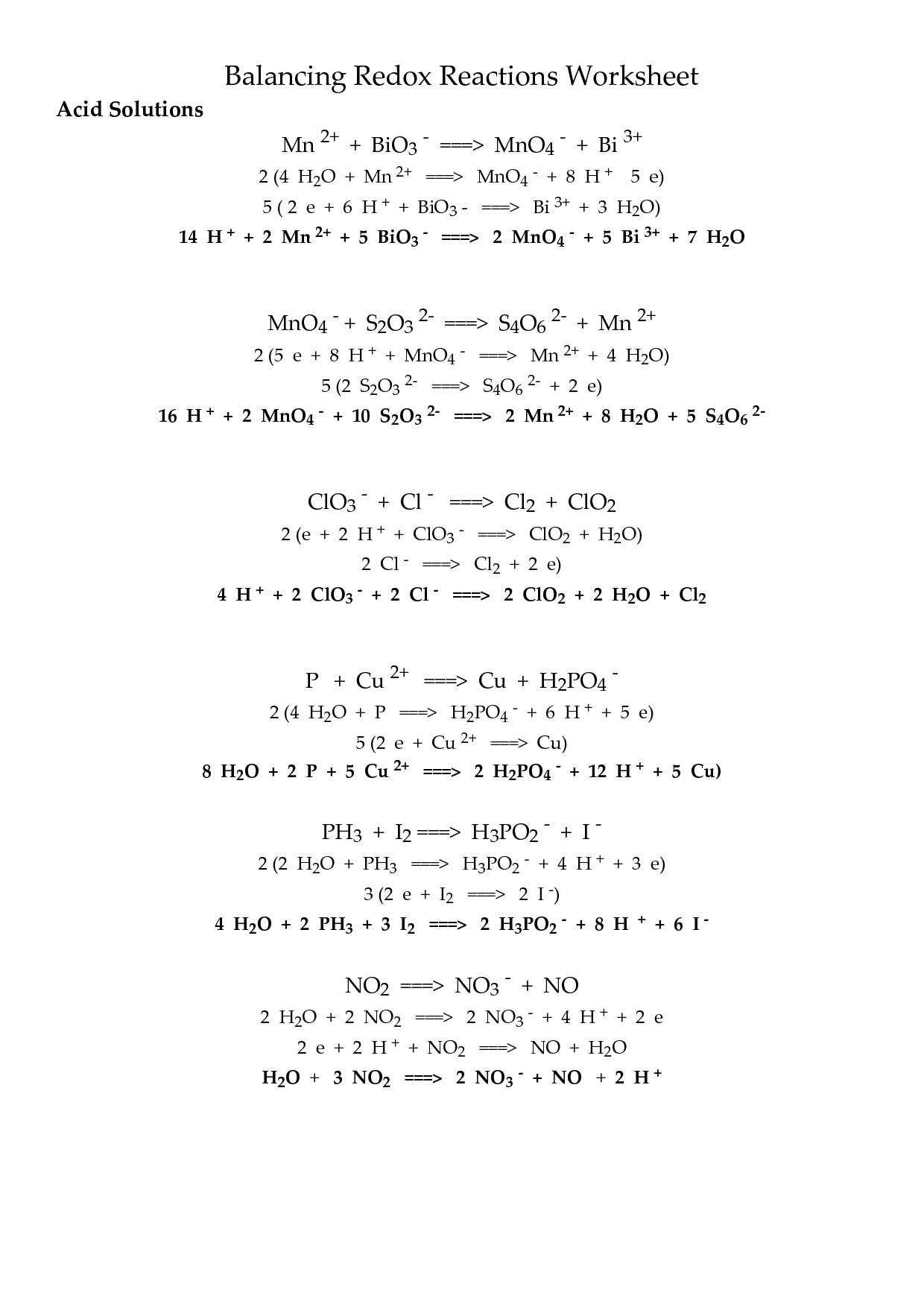





























Comments