Organic Molecules Functional Groups Worksheet
Are you a high school or college student studying chemistry? If so, you may find yourself in need of some extra practice with identifying and understanding different functional groups in organic molecules. This worksheet is designed to help you fine-tune your knowledge and skills in this area, providing detailed examples and explanations to ensure your success in this important topic.
Table of Images 👆
- Organic Chemistry Functional Groups
- Functional Groups Worksheet
- Chemistry Functional Group Worksheets
- Polar and Nonpolar Functional Groups
- Organic Compounds with Functional Groups
- Organic Chemistry Nomenclature Worksheets with Answers
- Organic Chemistry Functional Groups Table
- Carbon Compounds Worksheet
- Organic Chemistry Functional Groups
- Organic Chemistry Functional Groups Worksheet
- Organic Chemistry Functional Groups Worksheet
- Chemistry Functional Group Worksheets
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
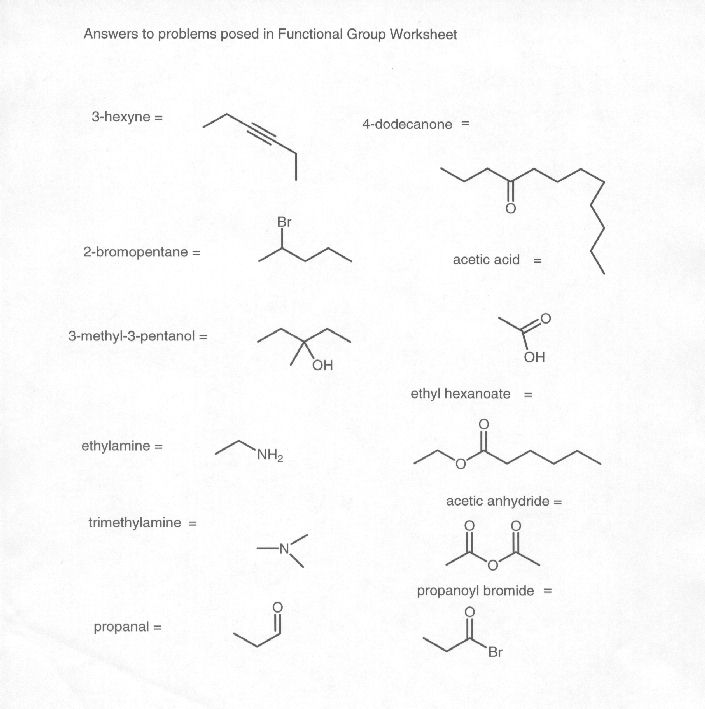
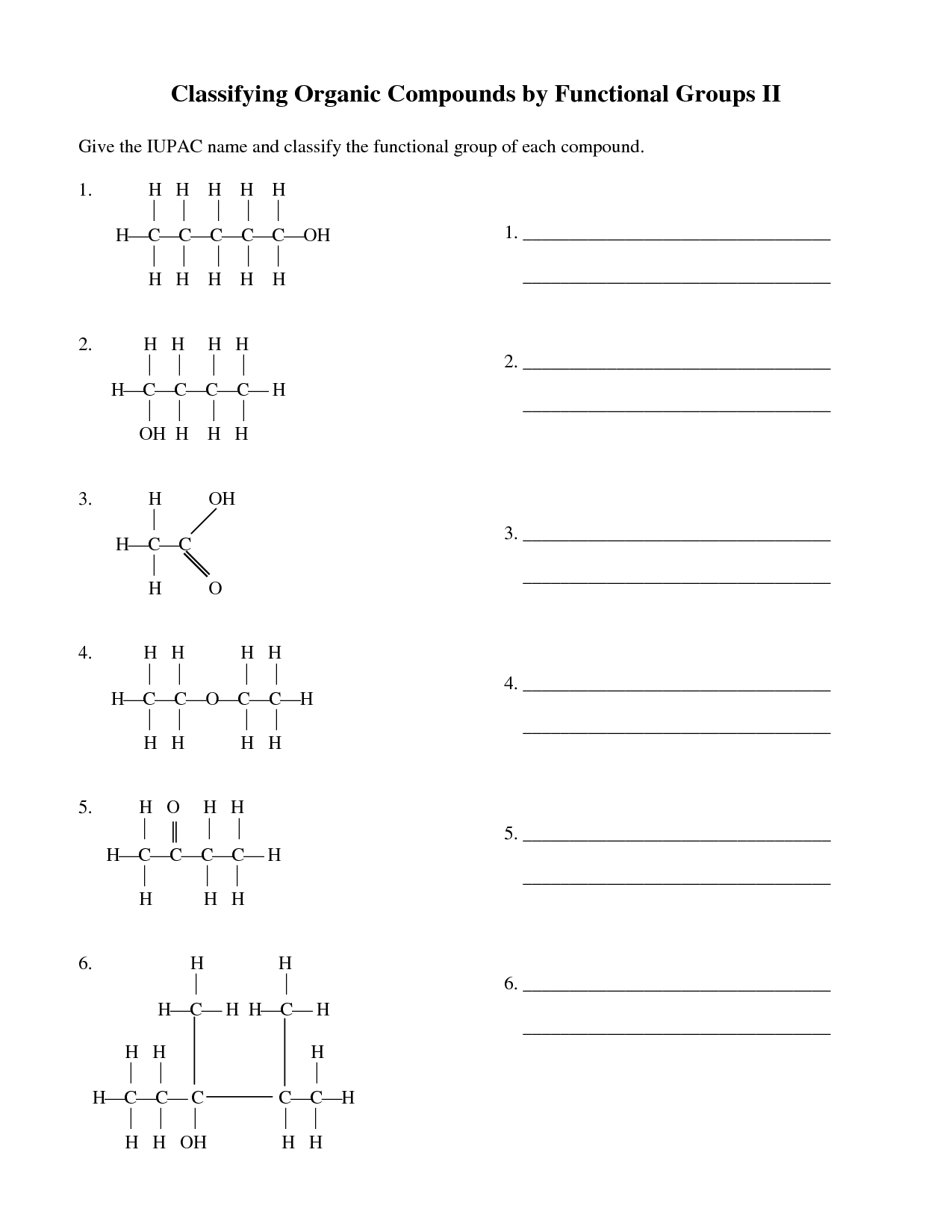
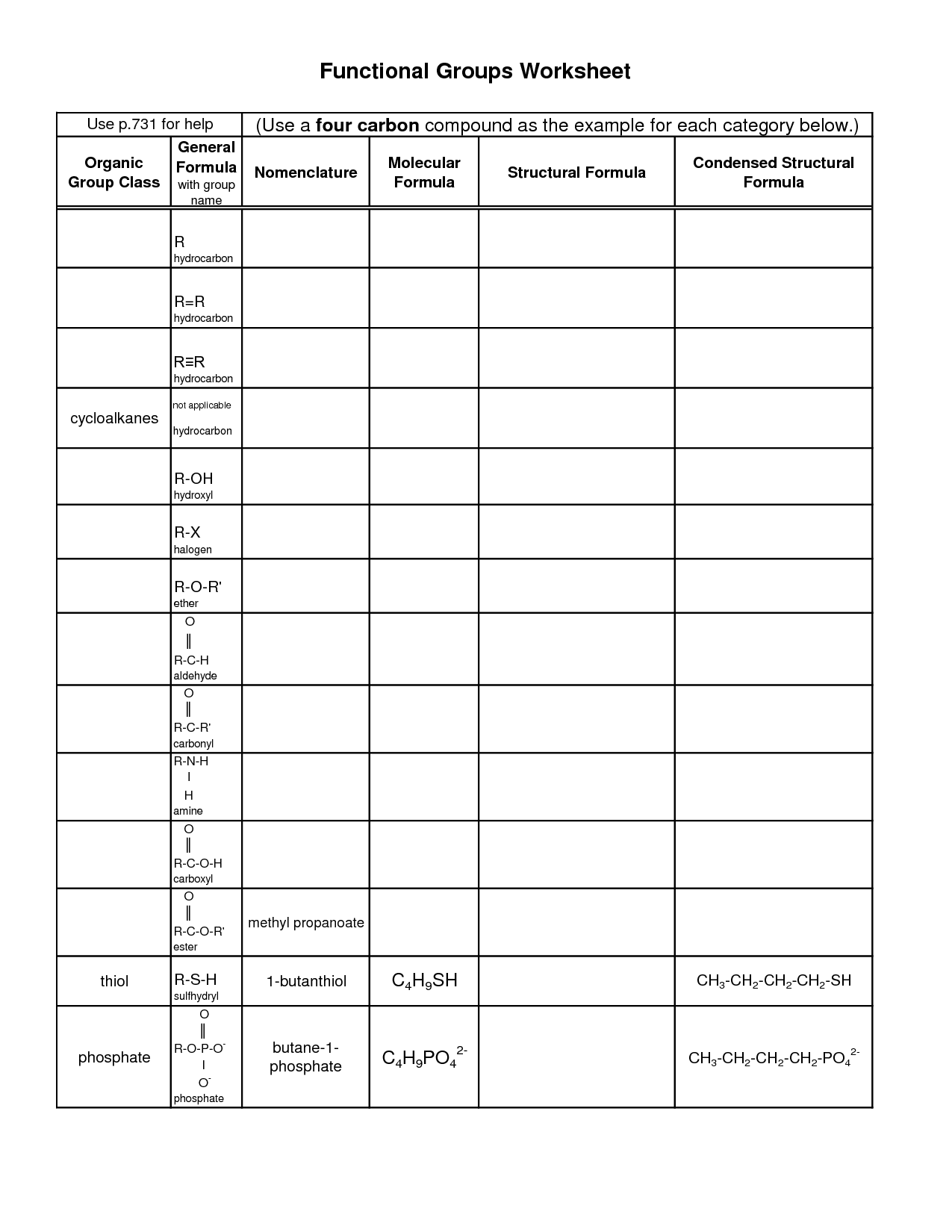
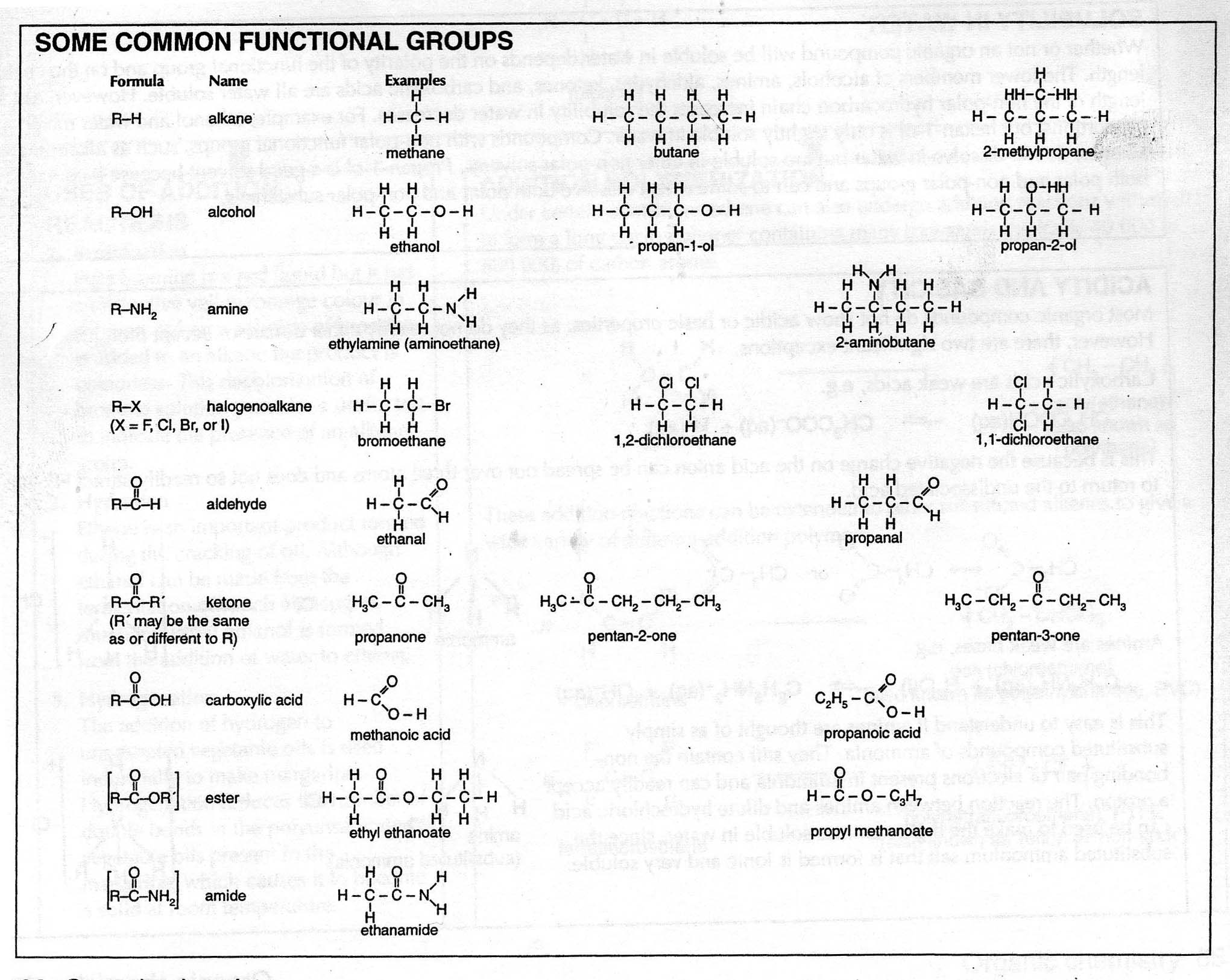
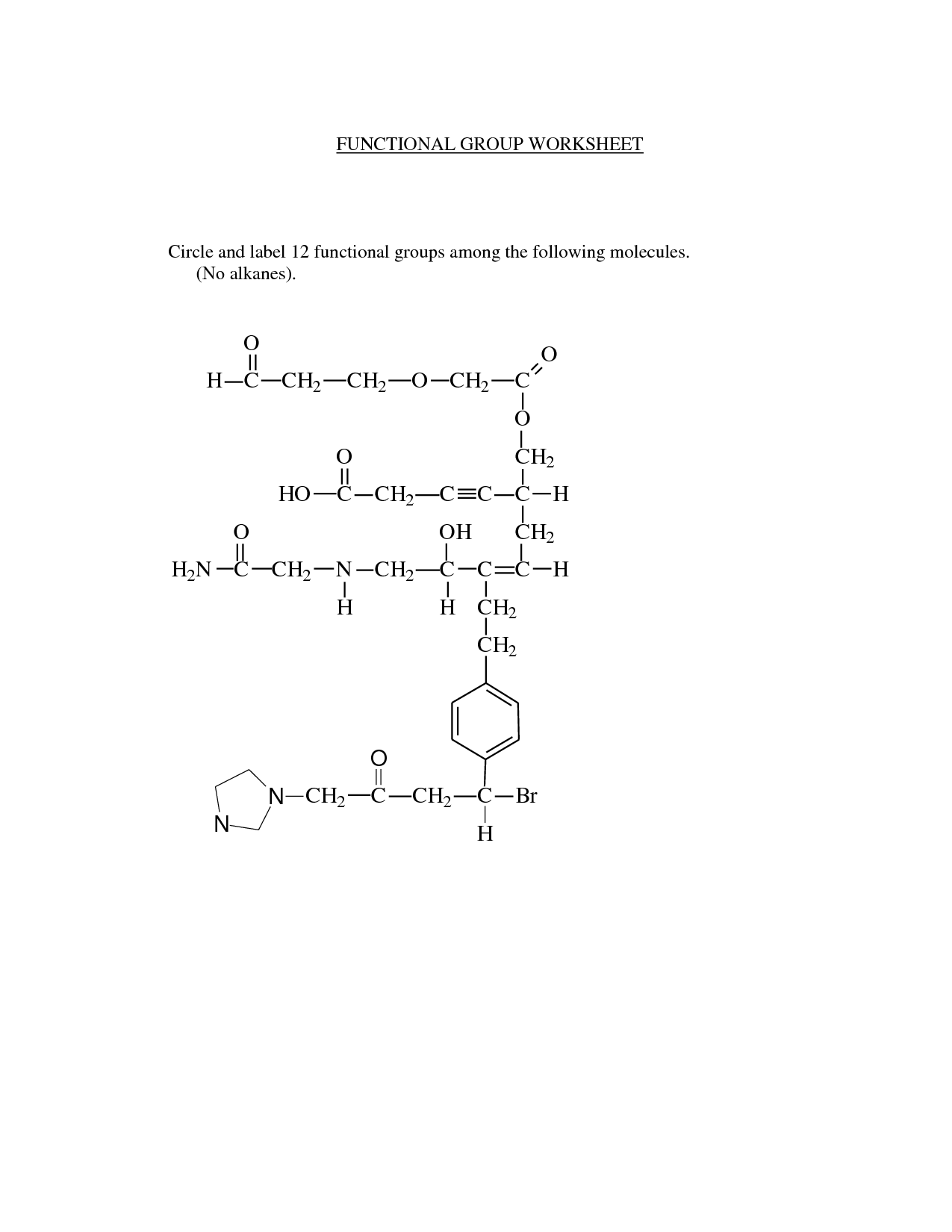
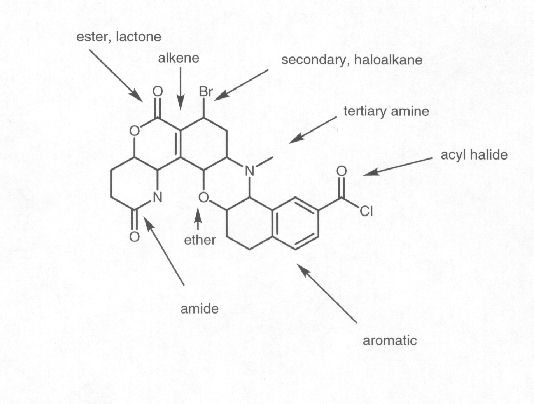
What is a functional group?
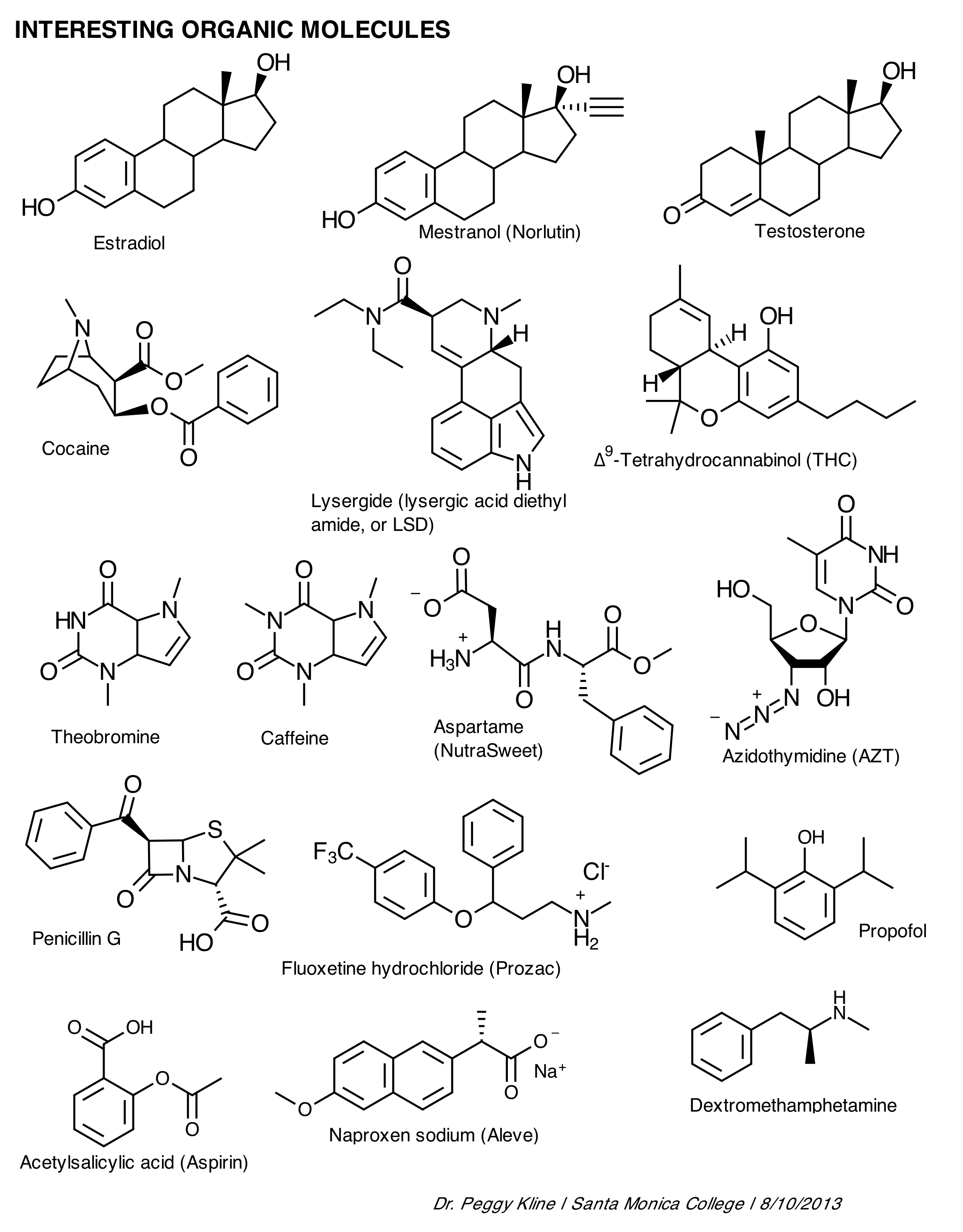
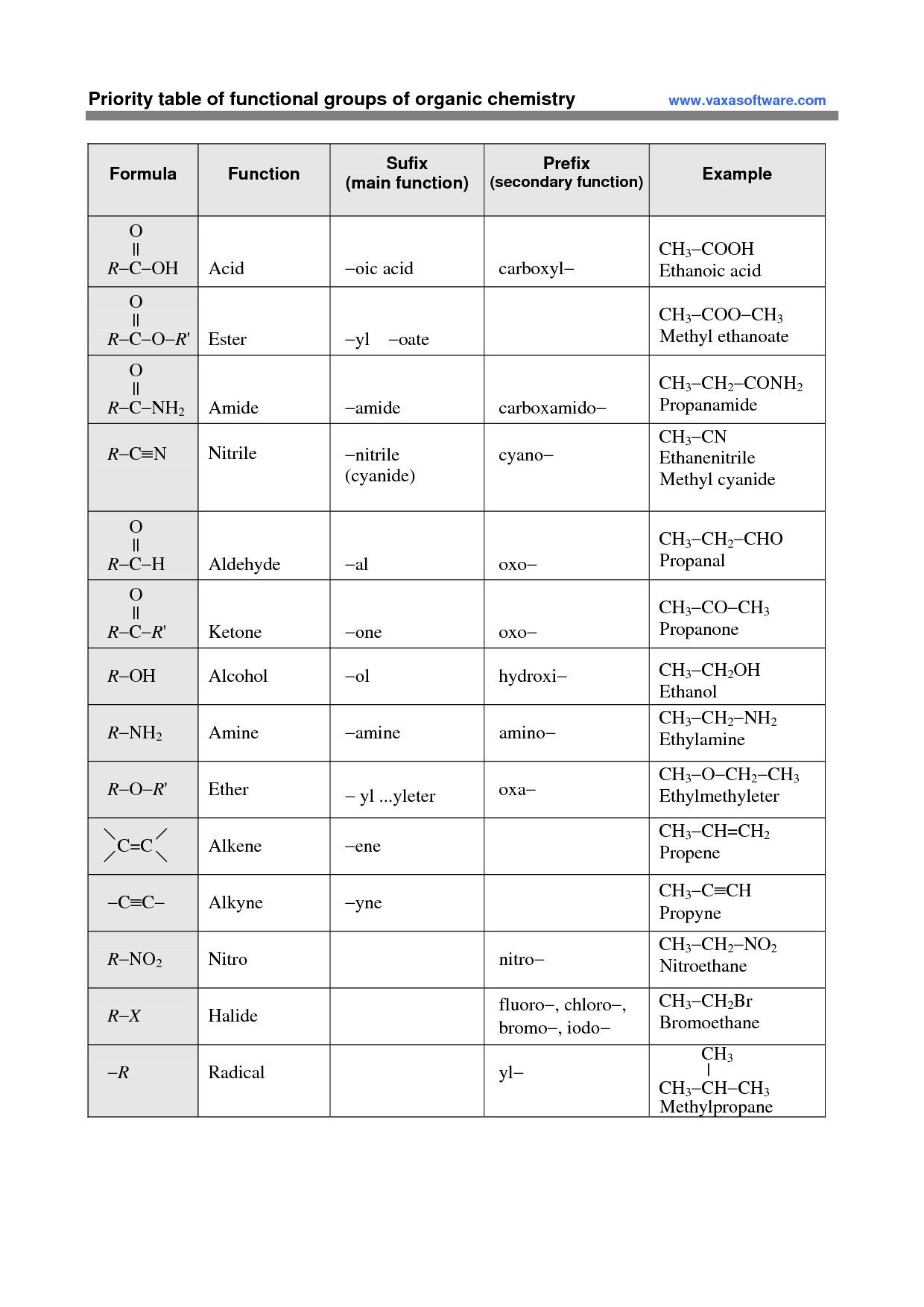
A functional group is a specific group of atoms within a molecule that determines the chemical properties and reactivity of that molecule. Functional groups are responsible for the characteristic chemical behavior of organic compounds and play a crucial role in defining the structure and function of the molecule. Common functional groups include hydroxyl (-OH), carbonyl (-C=O), and amino (-NH2) groups, among others.
What is the functional group present in alcohols?
The functional group present in alcohols is the hydroxyl group (-OH), which consists of an oxygen atom bonded to a hydrogen atom that is attached to a carbon atom in the alkyl chain of the alcohol molecule. This hydroxyl group is what characterizes alcohols and gives them their unique chemical properties, such as the ability to participate in reactions like oxidation and esterification.
What functional group is found in carboxylic acids?
The functional group found in carboxylic acids is the carboxyl group, which consists of a carbonyl group (C=O) bonded to a hydroxyl group (–OH) attached to the same carbon atom.
Which functional group is responsible for the unique properties of amines?
The amino group (-NH2) is the functional group responsible for the unique properties of amines. Amines have a characteristic ammonia-like odor and are basic in nature due to the lone pair of electrons on the nitrogen atom, which can accept a proton to form an ammonium ion. Additionally, amines can participate in nucleophilic reactions due to the lone pair of electrons, making them versatile compounds in organic chemistry.
What functional group is present in aldehydes and ketones?
The functional group present in aldehydes and ketones is the carbonyl group, which is composed of a carbon atom double-bonded to an oxygen atom.
What is the functional group that characterizes ethers?
The functional group that characterizes ethers is the oxygen atom bonded to two carbon atoms in an R-O-R' structure, where R and R' represent alkyl or aryl groups. This oxygen atom is the defining feature of ethers and is responsible for their unique chemical properties and reactivity.
Which functional group is found in thioalcohols?
Thioalcohols contain the thiol functional group, which is characterized by a sulfur atom bonded to a hydrogen atom and an alkyl or aryl group. Thioalcohols are analogs of alcohols where an oxygen atom in the hydroxyl group of the alcohol is replaced by a sulfur atom.
What functional group is present in esters?
The functional group present in esters is the carbonyl group (-C=O) attached to an oxygen atom that is also bonded to an alkyl or aryl group.
Which functional group is responsible for the acidic nature of carboxylic acids?
The carboxyl group (-COOH) is responsible for the acidic nature of carboxylic acids. This group can release a hydrogen ion (H+) in solution, resulting in the formation of carboxylate anion and creating acidity in solution.
What functional group is found in amides?
The functional group found in amides is the carbonyl group, which is characterized by a carbon atom double bonded to an oxygen atom. In amides, this carbonyl group is connected to a nitrogen atom, forming the -CONH2 structure.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





















Comments