Mole Conversion Worksheet Answer Key
Are you a student struggling with mole conversions in chemistry? Look no further! This blog post will provide you with a mole conversion worksheet answer key to help you better understand this important topic. By breaking down each step and providing clear explanations, this worksheet will assist you in mastering mole conversions and achieving success in your chemistry studies.
Table of Images 👆
- Mole Ratio Worksheet Answers
- Convert Grams to Moles
- Mole Ratio Worksheet Answer Key
- Mass to Mole Stoichiometry Worksheet Answer Key
- Mole Molecules and Grams Worksheet Answer Key
- Moles and Mass Worksheet Answers
- Mole Conversion Worksheet Answers
- Mole Conversion Worksheet
- Mole Conversion Worksheet Answers
- Dimensional Analysis Worksheet Answer Key
- Mole Conversion Worksheet Answers
- Mole Ratio Worksheet Answer Key
- Chemistry Mole Conversion Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is a mole conversion?
A mole conversion is a mathematical process used to convert the quantity of a substance from one unit to another, typically involving the number of moles of that substance. This conversion allows chemists to compare and communicate quantities of different substances in chemical reactions, making it a fundamental concept in chemistry for calculating amounts of reactants and products in reactions.
What is the purpose of a mole conversion worksheet?
The purpose of a mole conversion worksheet is to practice converting between moles, number of particles, mass, volume, and concentration of substances in chemistry. It helps students develop their understanding of the concept of the mole and its applications in chemical calculations, ensuring they can accurately convert between different units and quantities in various types of problems.
How do you convert moles to grams?
To convert moles to grams, you need to multiply the number of moles by the molar mass of the substance. The molar mass is found by adding up the atomic masses of all the elements in the compound, as given in the periodic table. This calculation allows you to determine the weight of the substance in grams.
How do you convert grams to moles?
To convert grams to moles, you need to divide the given mass in grams by the molar mass of the substance in grams per mole. The molar mass is obtained by summing the atomic masses of all the elements present in the compound, which can be found on the periodic table. This calculation allows you to determine the number of moles present in a given mass of a substance.
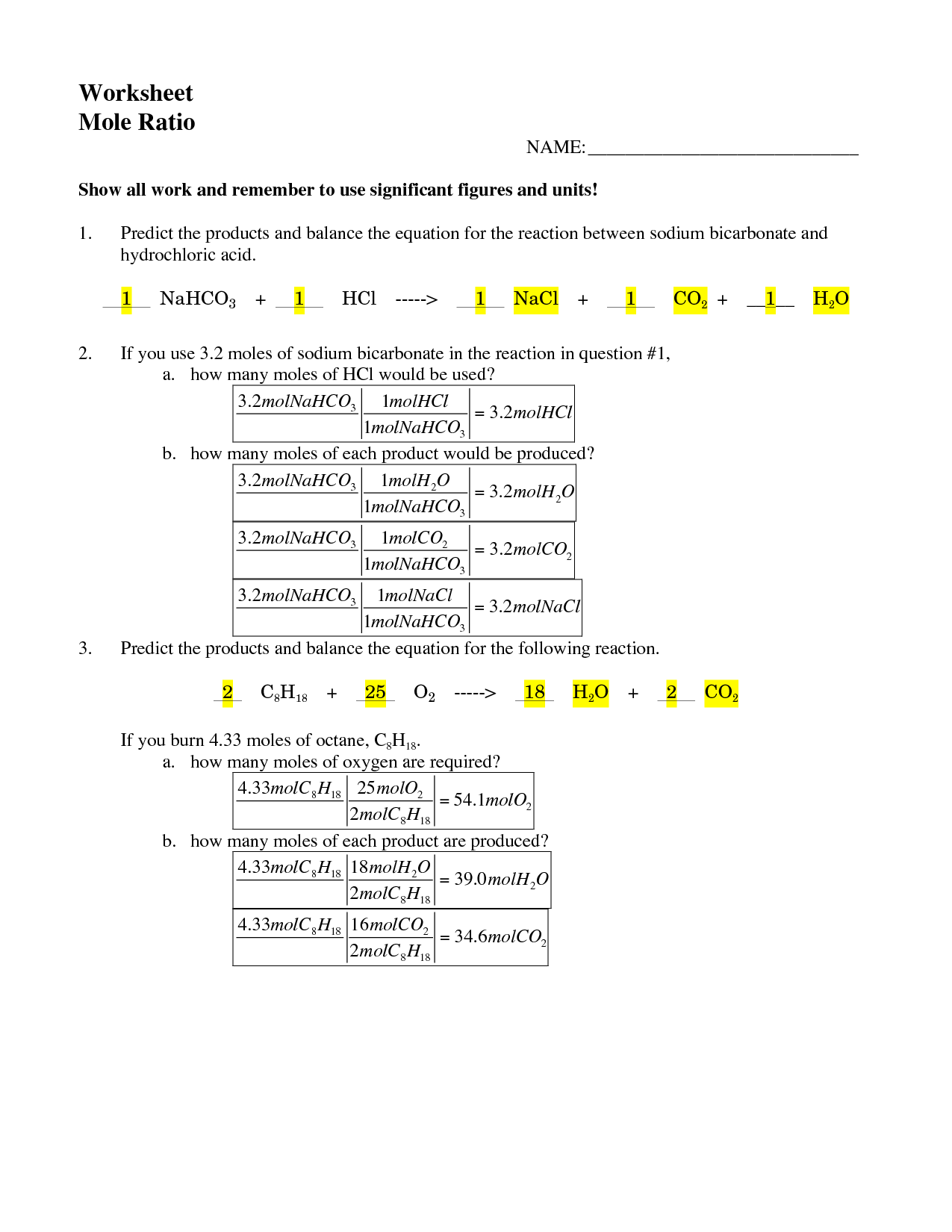
What is the mole ratio in a chemical equation?
The mole ratio in a chemical equation refers to the ratio of moles of one substance to another in a balanced chemical equation. It indicates the number of moles of each reactant and product involved in a reaction and is used to determine the stoichiometry of the reaction, allowing for the calculation of the quantities of substances consumed and produced in the reaction.
How do you use the mole ratio to convert between different substances?
To use the mole ratio to convert between different substances, you first need to balance the chemical equation to determine the ratio of moles between the substances involved. Then, you can use this ratio as a conversion factor in stoichiometric calculations. Simply multiply the given amount of one substance by the mole ratio from the balanced equation to find the amount of the other substance produced or consumed. This method allows you to relate the amounts of different substances in a chemical reaction based on their molar quantities.
What is Avogadro's number?
Avogadro's number is a constant representing the number of constituent particles, usually atoms or molecules, in one mole of a substance, which is approximately 6.022 x 10^23.
How is Avogadro's number used in mole conversions?
Avogadro's number (6.022 x 10^23) is used in mole conversions to convert between the mass of a substance and the number of atoms or molecules it contains. By using Avogadro's number, one can relate the number of moles of a substance to the number of atoms or molecules present in that sample, allowing for calculations involving stoichiometry, percent composition, and other chemical equations that involve the mole concept.
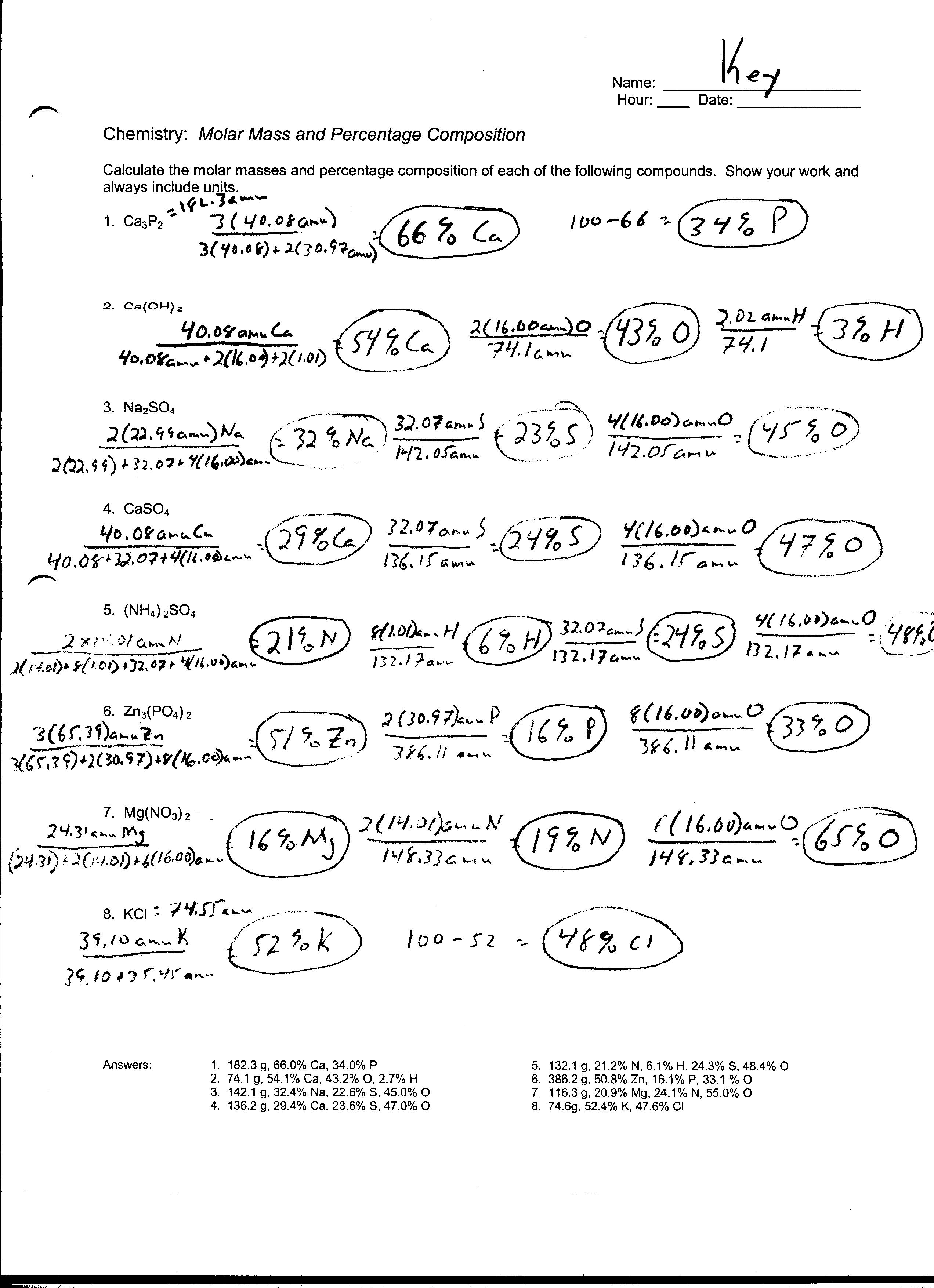
What is the molar mass of a substance?
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole. It is calculated by summing the atomic masses of all the atoms making up the molecule or formula unit of the substance. Molar mass is important in various chemical calculations, such as determining the amount of a substance needed for a reaction or calculating the percent composition of a compound.
How is the molar mass used in mole conversions?
The molar mass is used in mole conversions by providing the conversion factor between the mass of a substance and the number of moles of that substance. By multiplying the molar mass of a substance by the given mass in grams, you can convert the mass to moles. Conversely, by multiplying the molar mass by the number of moles, you can convert moles to mass. This helps in calculations involving the amount of a substance in chemical reactions or other chemical processes.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments