High School Periodic Table Worksheets
If you’re a high school student hoping to deepen your understanding of the periodic table, then you’ve come to the right place. These periodic table worksheets are designed to help you grasp the concepts of elements and their properties, making your study sessions more engaging and effective. Whether you’re learning about the periodic table for the first time or need a refresher, these worksheets will provide you with a solid foundation to build upon.
Table of Images 👆
- Periodic Table Labeling Worksheet
- Blank Periodic Table
- Periodic Table Worksheets Middle School
- Periodic Table Worksheets
- Electron Configuration Worksheet Answer Key
- Periodic Table Worksheet Answer Key
- Naming Covalent Compounds Worksheet
- Periodic Table Worksheet Fill in Blank
- All About Me Writing
- Constitution Scavenger Hunt Answer Key AP Government
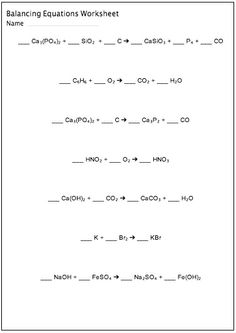
- Balancing Chemical Equations Worksheet Answers
- Third Grade Sight Words Printable
- Third Grade Sight Words Printable
- Third Grade Sight Words Printable
- Third Grade Sight Words Printable
- Third Grade Sight Words Printable
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is the periodic table?
The periodic table is a tabular arrangement of chemical elements organized by their atomic number, electron configurations, and recurring chemical properties. It displays elements in rows and columns based on their characteristics, with elements exhibiting similar properties positioned together. This table is a fundamental tool in chemistry, allowing scientists to predict the behavior and properties of elements based on their placement and relationships within the table.
Who developed the periodic table?
The periodic table was developed by Russian chemist Dmitri Mendeleev in 1869. He organized the elements based on their atomic mass and properties, creating a systematic way to understand and categorize the elements according to their chemical behaviors.
How is the periodic table organized?
The periodic table is organized by arranging elements in order of increasing atomic number, with elements having similar properties placed in columns known as groups. The rows, known as periods, represent increasing numbers of electron shells. The periodic table is divided into metals, metalloids, and nonmetals, and elements are grouped based on similar chemical properties and atomic structure.
What do the rows in the periodic table represent?
The rows in the periodic table, also known as periods, represent the number of electron shells in an atom's structure. Each row signifies a new energy level or shell that an element's electrons occupy, with elements in the same row sharing similar electron configurations and chemical properties.
What are the columns in the periodic table called?
The columns in the periodic table are called groups or families.
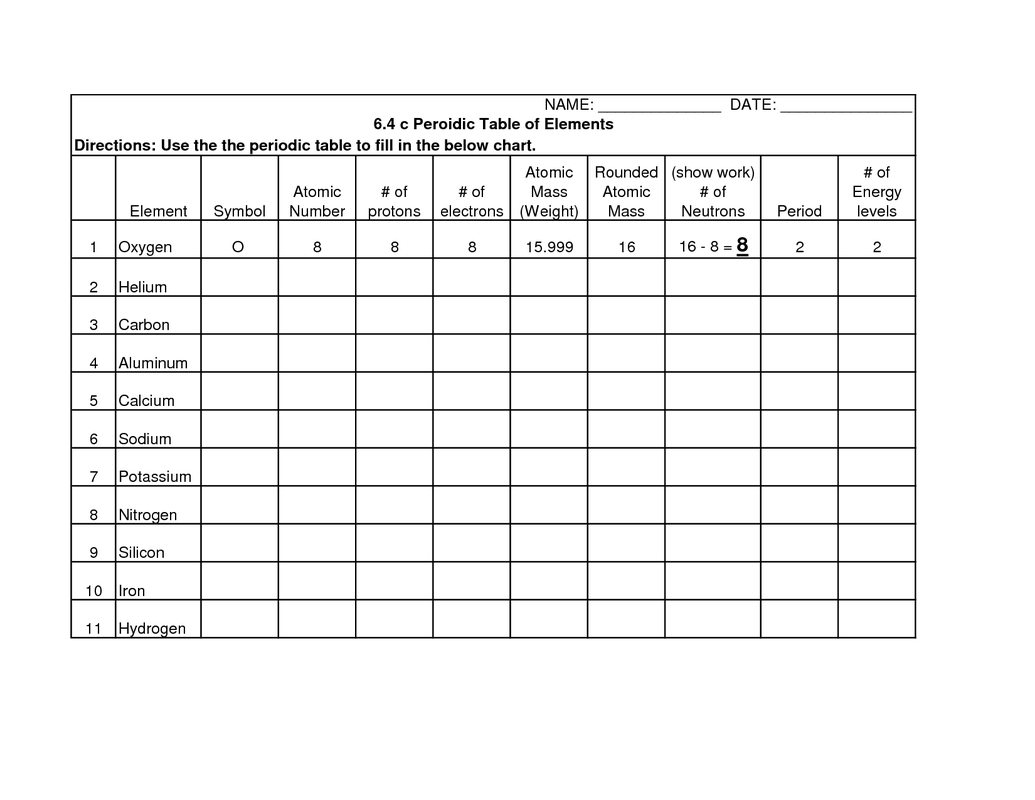
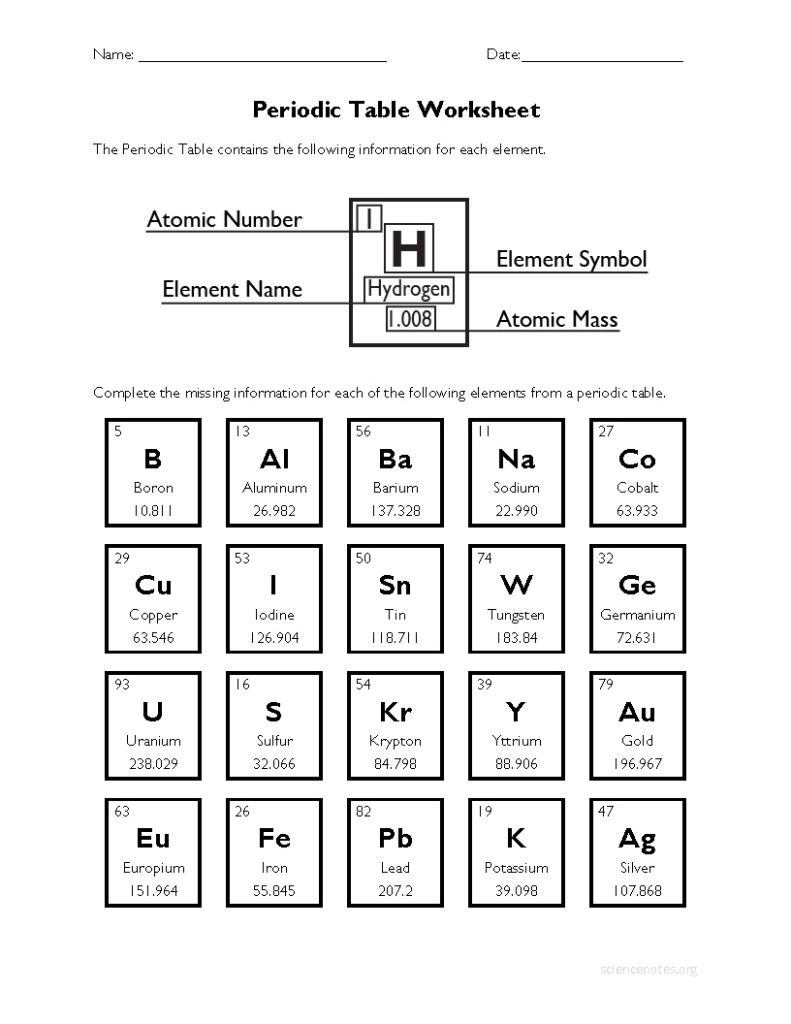
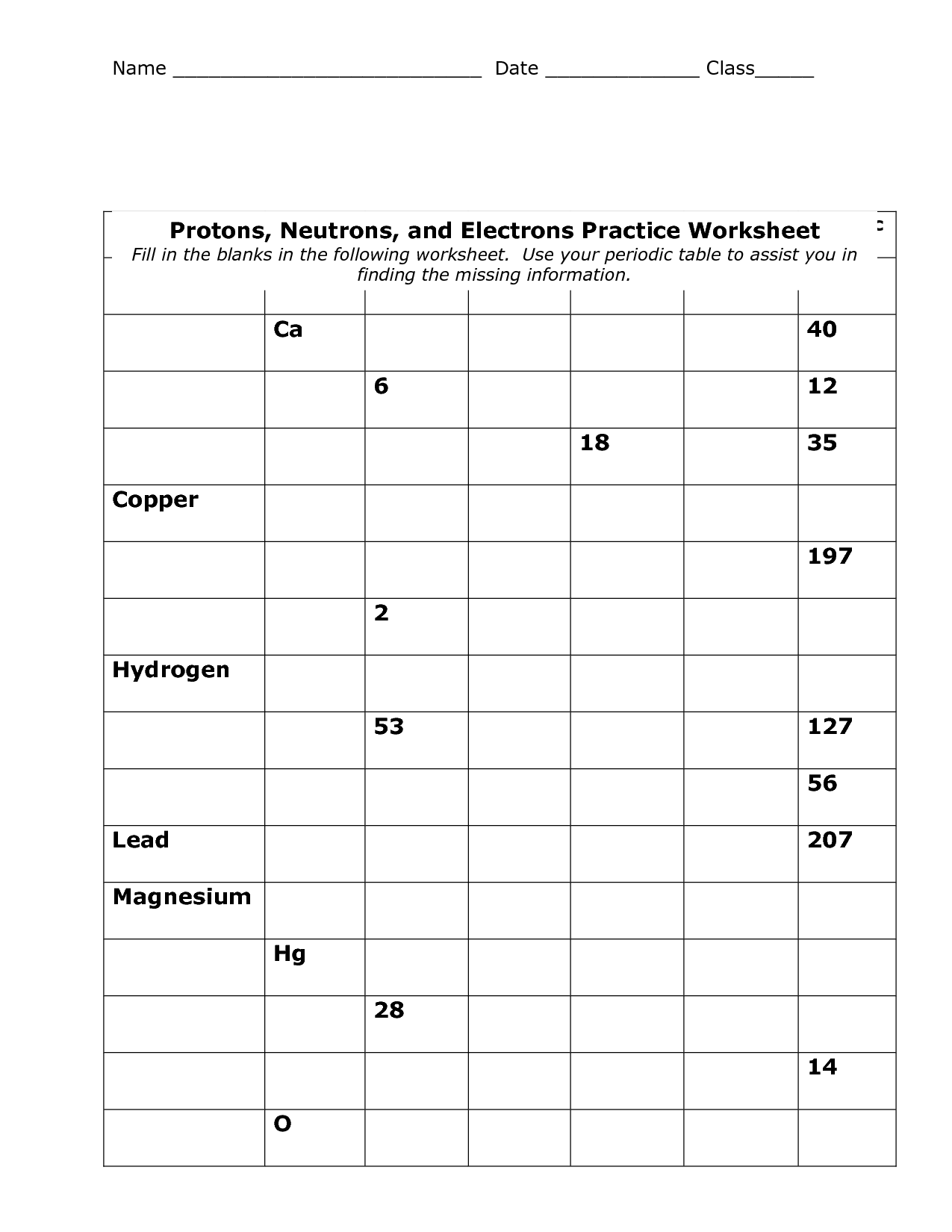
What information does each element's square on the periodic table provide?
Each element's square on the periodic table provides information such as the element's atomic number, symbol, atomic mass, and the element's name. This information helps to identify and organize the elements based on their properties and characteristics.
What is atomic number?
The atomic number is the number of protons found in the nucleus of an atom. It is a unique identifier for each element on the periodic table, as no two elements have the same number of protons. The atomic number determines the chemical properties and placement of an element within the periodic table.
What is atomic mass?
Atomic mass is the average mass of an atom of an element, taking into account the relative abundance of each isotope of that element. It is measured in atomic mass units (amu) and is typically reported on the periodic table underneath the element's symbol.
How does the periodic table help predict the properties of elements?
The periodic table helps predict the properties of elements by organizing them based on their atomic number, which reflects the number of protons in their nucleus. Elements within the same group tend to have similar chemical properties because they have the same number of valence electrons, which are responsible for an element's chemical behavior. Additionally, elements become more metallic as you move from right to left across a period and from top to bottom within a group on the periodic table, helping to predict trends in properties such as reactivity, electronegativity, and atomic size.
How is the periodic table used in chemistry and science?
The periodic table is a fundamental tool used in chemistry and science to organize and classify elements based on their properties and characteristics. It provides a systematic way of understanding the relationships between different elements, their chemical reactivity, electron configurations, and physical properties. By utilizing the periodic table, scientists can predict and explain the behavior of elements, as well as make informed decisions about reactions, compounds, and materials in various fields such as research, industry, and education.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






























Comments