GED Chemistry Worksheet
Worksheets are an invaluable tool for those seeking to practice and improve their understanding of GED Chemistry concepts. When it comes to finding a reliable resource to enhance your learning experience in this subject, worksheets offer a structured format designed to engage and challenge your understanding of important topics.
Table of Images 👆
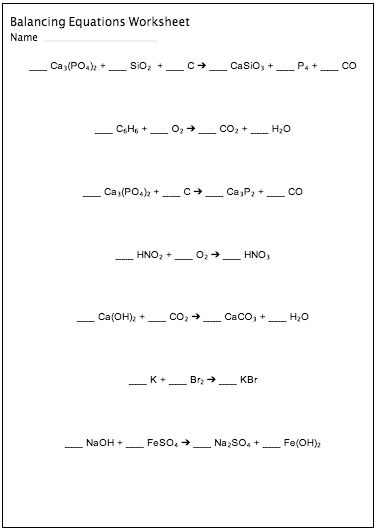
- Practice Balancing Chemical Equations Worksheet
- Free Printable GED Practice Test Worksheets
- Social Studies GED Practice Test and Answers
- College Business Math Word Problems Worksheets
- Glencoe Science Worksheet Answer Key
- Glencoe Chemistry Chapter Assessment Answers
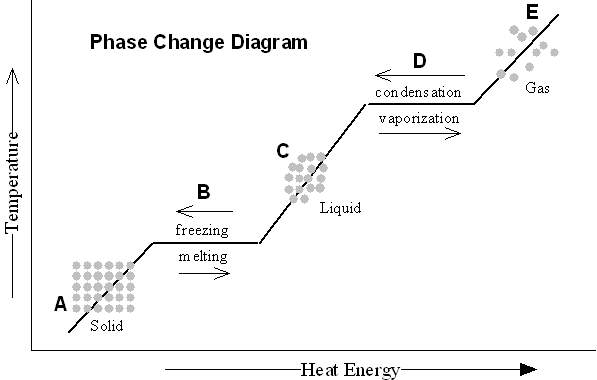
- Blank Phase Change Diagram
- Convert Improper Fractions to Mixed Numbers
- Balancing Chemical Equations Worksheet
- Math U See Worksheet Generator
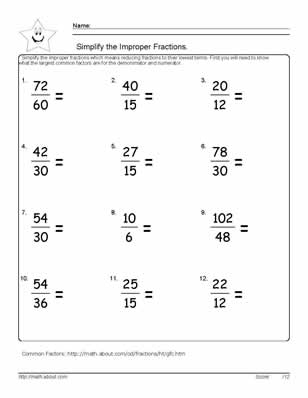
- Simplify Improper Fractions Worksheet
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Stoichiometry Worksheet Answer Key
Chemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is a chemical equation?
A chemical equation is a symbolic representation that shows the reactants and products involved in a chemical reaction. It uses chemical formulas and symbols to depict the substances participating in the reaction and the changes that occur as a result of the reaction. The equation must follow the law of conservation of mass, meaning that the number and type of atoms on the reactant side must be equal to the number and type of atoms on the product side.
What are the differences between physical and chemical changes?
Physical changes involve a change in a substance's physical properties, such as shape, size, or phase (solid, liquid, gas), without altering its chemical composition. Examples include melting ice or boiling water. On the other hand, chemical changes involve a rearrangement of atoms and result in the formation of a new substance with different chemical properties. This includes processes like rusting of iron or burning of wood.
How do you calculate the molar mass of a compound?
To calculate the molar mass of a compound, you add up the atomic masses of all the elements in the compound based on the chemical formula. This involves multiplying the atomic mass of each element by the number of atoms of that element in the compound, and then summing up the results. The units used are in grams per mole (g/mol), which represents the molar mass of the compound.
What is an acid and a base? Provide examples.
Acids are substances that donate protons in a chemical reaction, increasing the concentration of hydrogen ions in a solution. Examples of acids include hydrochloric acid (HCl) found in the stomach, citric acid found in citrus fruits, and acetic acid found in vinegar. Bases, on the other hand, are substances that accept protons or donate hydroxide ions, decreasing the concentration of hydrogen ions in a solution. Examples of bases include sodium hydroxide (NaOH) found in drain cleaner, ammonia found in household cleaners, and sodium bicarbonate found in baking soda.
Discuss the concept of valence electrons in relation to chemical bonding.
Valence electrons are the electrons in the outermost energy level of an atom that are involved in bonding with other atoms. These electrons determine the reactivity and chemical behavior of an element. When atoms bond to form molecules, they interact to either gain, lose, or share electrons in order to attain a full outer electron shell and achieve a stable configuration. This process, known as chemical bonding, can result in the formation of ionic, covalent, or metallic bonds depending on how the valence electrons are shared or transferred between atoms. Overall, the concept of valence electrons plays a crucial role in understanding the nature of chemical bonding and the properties of different compounds.
Explain the Law of Conservation of Mass and its significance in chemical reactions.
The Law of Conservation of Mass states that in a closed system, mass can neither be created nor destroyed, but only transferred or converted from one form to another. This law is significant in chemical reactions because it ensures that the total mass of reactants must equal the total mass of products. In other words, the atoms present at the beginning of a reaction must be accounted for in the products formed. This principle is a fundamental concept in chemistry and allows scientists to predict and understand the outcomes of chemical reactions.
Describe the properties and uses of acids, bases, and salts.
Acids are substances that donate hydrogen ions in a solution, have a sour taste, and turn blue litmus paper red. Bases are substances that accept hydrogen ions, have a bitter taste, and turn red litmus paper blue. Salts are compounds formed from the reaction between an acid and a base, have a neutral pH, and can be used in various applications such as food preservation, cleaning, and industrial processes. Acids are commonly used in industries like pharmaceuticals and food production, bases are used in cleaning products and agriculture, and salts have uses ranging from food seasoning to water softening.
What is the difference between an ionic and a covalent bond?
An ionic bond is formed when electrons are transferred between atoms, resulting in the attraction between oppositely charged ions. This bond occurs between a metal and a nonmetal, leading to the formation of a crystal lattice structure. In contrast, a covalent bond is formed when atoms share electrons to achieve a stable electron configuration. This bond typically occurs between nonmetals and results in the formation of molecules. In summary, the main difference is that an ionic bond involves electron transfer, while a covalent bond involves electron sharing.
How can you determine the empirical formula of a compound using percentage composition?
To determine the empirical formula of a compound using percentage composition, you first convert the percentage of each element in the compound into grams. Then, divide the grams of each element by its molar mass to find the moles of each element present. Next, determine the simplest whole number ratio of the moles of each element by dividing each by the smallest number of moles obtained. Finally, write the chemical formula using these whole number ratios as subscripts, which gives you the empirical formula of the compound.
Discuss the concepts of exothermic and endothermic reactions and provide examples.
Exothermic reactions release energy in the form of heat to the surroundings, resulting in a temperature increase. An example of an exothermic reaction is the combustion of gasoline in a car engine. On the other hand, endothermic reactions absorb energy from the surroundings, causing a temperature decrease. An example of an endothermic reaction is the dissolution of ammonium nitrate in water, which absorbs heat from the surroundings, making the solution feel colder.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.








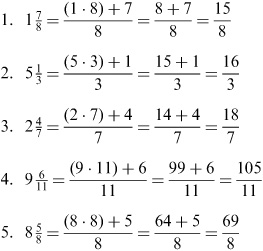












Comments