Energy Levels Worksheet
Are you an educator or a student looking for a helpful resource to better understand energy levels? Look no further! This blog post will introduce you to a handy energy levels worksheet that will facilitate your learning and comprehension of this essential subject.
Table of Images 👆
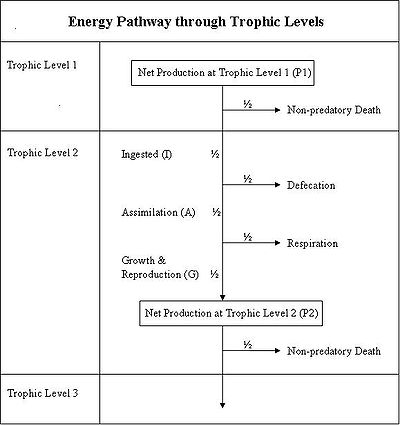
- Energy Flow through Ecosystem Worksheet
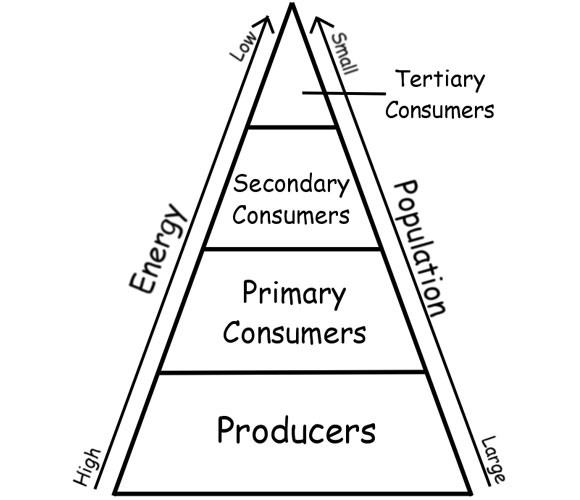
- Science Energy Pyramid
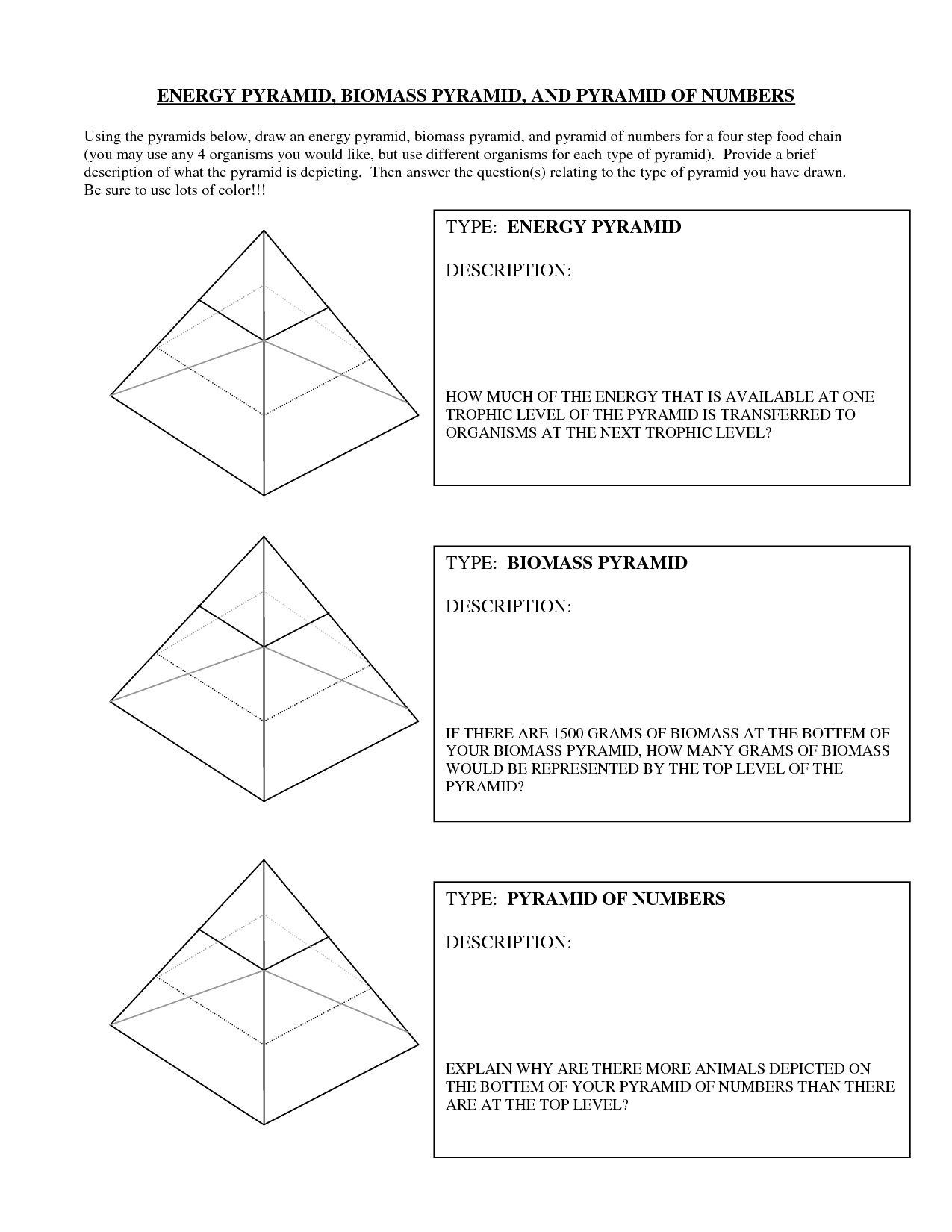
- Biomass Energy Pyramid Worksheet
- Food Web Energy Pyramid Worksheet
- POGIL Biology Answer Key
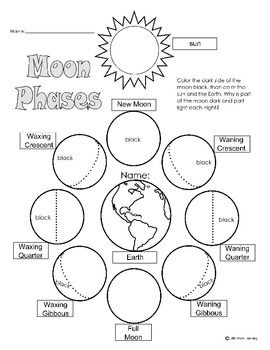
- Moon Phases Worksheet
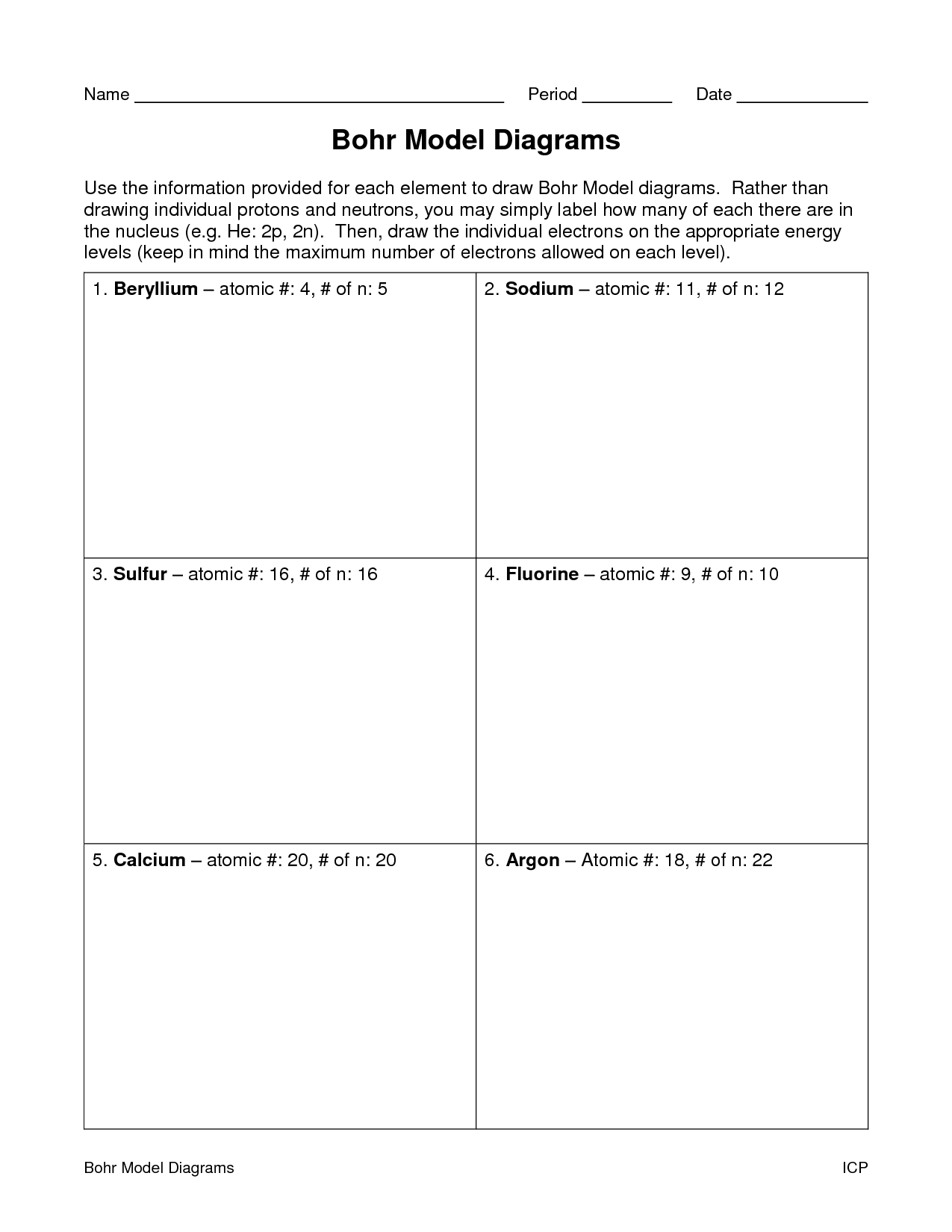
- Blank Bohr Model Worksheet
- Energy Flow through Ecosystem Worksheet Answers
- Toy Story Printable Worksheets
- Human Digestive System Worksheet Answers
More Energy Worksheets
Light and Heat Energy WorksheetsTypes of Energy Transfer Worksheet
Energy Light Heat Sound Worksheets
3 Forms of Energy Worksheets
Types of Energy Worksheet PDF
Energy Worksheets for Third Grade
What are energy levels?
Energy levels, in the context of atoms and molecules, refer to the different possible quantized energies that an electron can occupy within an atom. These energy levels are defined by the amount of energy required to move an electron from one level to another, and they determine the ways in which atoms interact with each other through the exchange and sharing of energy. The arrangement of electrons in these energy levels plays a crucial role in understanding the chemical properties and behavior of elements and compounds.
How are energy levels of electrons in an atom defined?
The energy levels of electrons in an atom are defined by the specific amount of energy that an electron can have within the atom. These energy levels are quantized and discrete, meaning that electrons can only occupy certain energy levels, known as orbitals, and cannot exist in between these levels. The energy levels are determined by the electron's distance from the nucleus and are organized into different shells and subshells. Electrons can move between these energy levels by absorbing or emitting energy, such as through the absorption or emission of photons.
How many energy levels are there in a hydrogen atom?
A hydrogen atom has a total of four energy levels: the ground state (n=1) and three excited states (n=2, n=3, and n=4).
What happens when an electron absorbs energy and moves to a higher energy level?
When an electron absorbs energy and moves to a higher energy level, it becomes excited and jumps to a shell farther away from the nucleus of an atom. This process is known as electron excitation. The electron will remain in this higher energy state temporarily before eventually emitting the absorbed energy in the form of a photon and returning to its original, lower energy level.
What happens when an electron releases energy and moves to a lower energy level?
When an electron releases energy and moves to a lower energy level, it emits a photon of light corresponding to the energy difference between the initial and final energy levels. This process is known as electron transition or electron relaxation and is a fundamental mechanism behind the emission of light in various phenomena such as atomic spectra and the operation of lasers.
How are energy levels organized in an atom?
Energy levels in an atom are organized into different electron shells, also known as energy levels or orbitals. These shells are numbered starting from the innermost shell with the lowest energy level. Electrons fill the inner shells first before moving to outer shells with higher energy levels. The number of electrons each shell can hold is determined by the energy level and follows a pattern based on the quantum mechanical properties of electrons.
What determines the energy difference between energy levels?
The energy difference between energy levels in an atom or molecule is primarily determined by the intrinsic properties of the system, such as the arrangement of electrons and the strength of the forces holding them in place. This energy difference is governed by principles of quantum mechanics, specifically the quantization of energy levels. The specific factors that influence the energy levels include the size of the nucleus, the number of protons and electrons, the orbital configuration, and the interactions between charged particles within the system. These factors collectively contribute to the unique energy levels and transitions observed in atoms and molecules.
How does the energy level of an electron affect its stability?
The energy level of an electron affects its stability by determining how tightly it is bound to the nucleus of an atom. Electrons with lower energy levels are closer to the nucleus and therefore experience stronger electrostatic forces, making them more stable since they are less likely to be easily removed or disrupted. On the other hand, electrons with higher energy levels are farther away from the nucleus and are therefore less stable, as they are more easily influenced by external factors and can be excited to higher energy levels or even leave the atom altogether.
How do electrons transition between energy levels?
Electrons transition between energy levels by absorbing or emitting photons of specific energies corresponding to the difference in energy levels. When an electron absorbs a photon, it moves to a higher energy level, and when it emits a photon, it moves to a lower energy level. This process follows the rules of quantum mechanics and is fundamental to understanding the behavior of electrons in atoms and molecules.
How do energy levels relate to the emission and absorption of light by atoms?
Energy levels of atoms determine the specific wavelengths of light that they can emit or absorb. When an electron in an atom absorbs energy, it jumps to a higher energy level. Upon returning to a lower energy level, the electron releases the excess energy in the form of light emission. The emitted light has a specific wavelength corresponding to the energy difference between the two levels. Similarly, when light of the correct wavelength interacts with an atom, it can be absorbed by an electron, causing it to move to a higher energy level. This relationship between energy levels and light emission/absorption forms the basis of spectral analysis and various technologies like spectroscopy.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.






















Comments