Elements Compounds and Mixtures Worksheet
A worksheet dedicated to exploring the fascinating world of elements, compounds, and mixtures awaits you. Whether you're an aspiring chemist, a curious student, or simply someone who enjoys expanding their scientific knowledge, this worksheet is designed to provide a comprehensive understanding of the entities and subjects within this captivating field.
Table of Images 👆
- Element Compound Mixture Worksheet
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
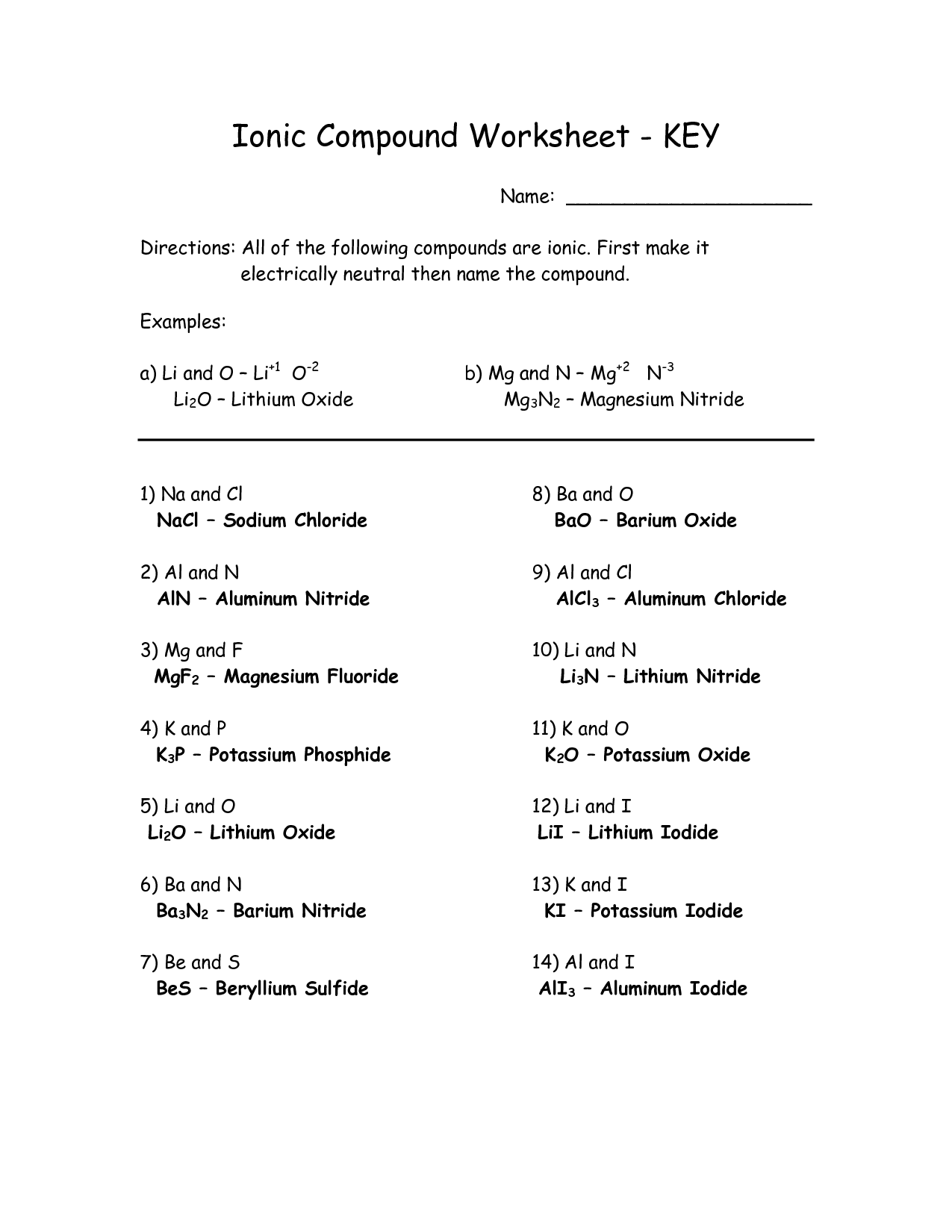
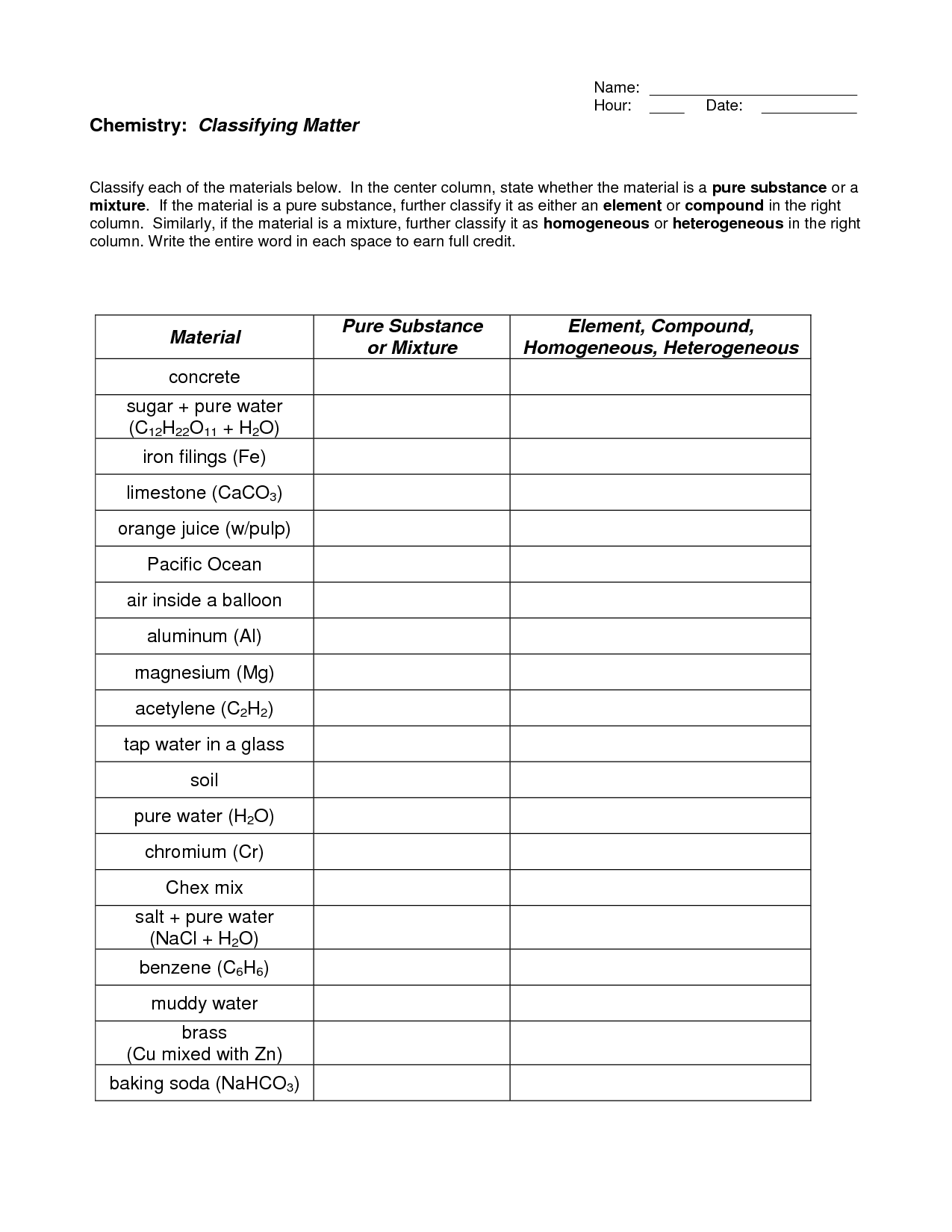
- Elements Compounds and Mixtures Worksheet Key
- Elements Compounds and Mixtures Worksheet Key
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
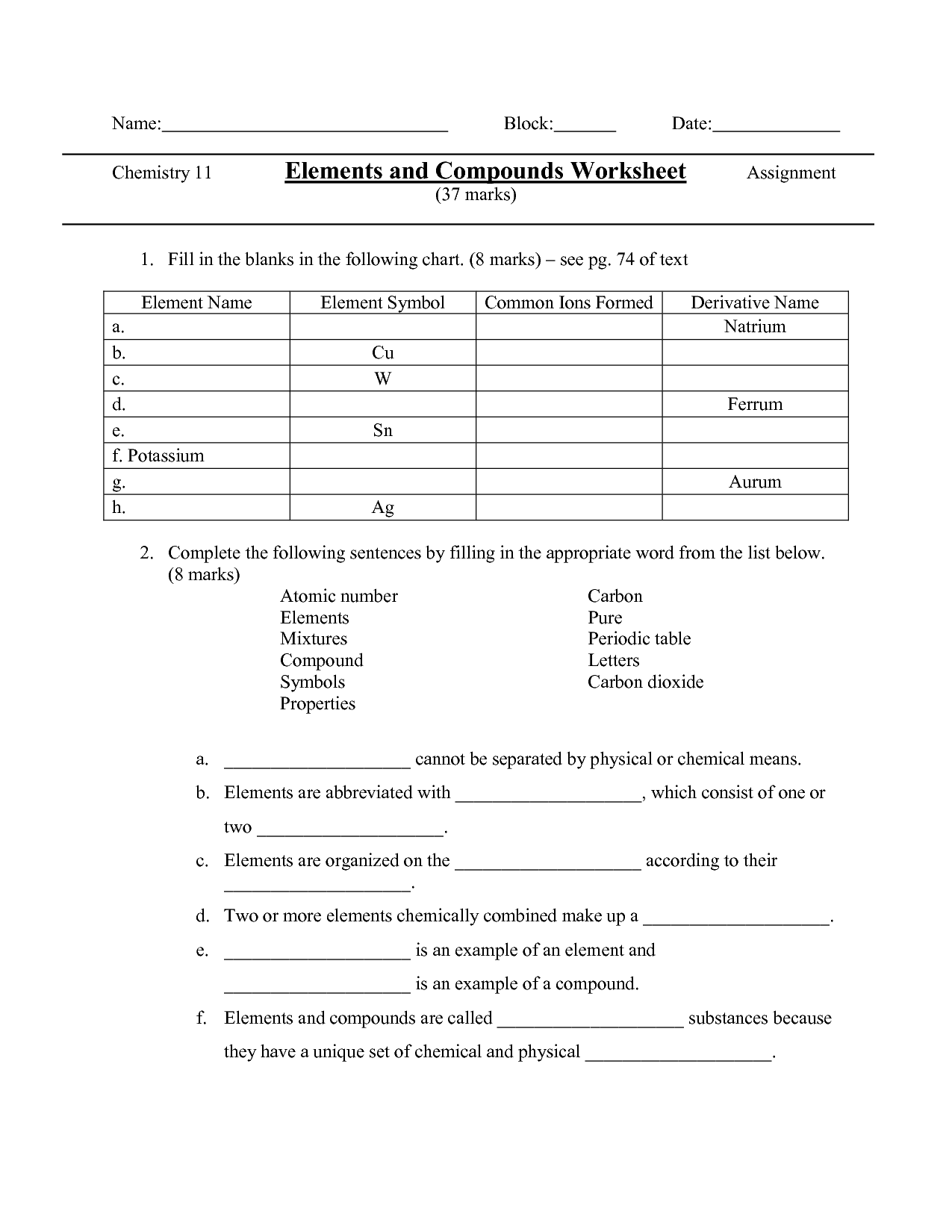
- Elements Compounds and Mixtures Worksheet Answer Key
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Elements Compounds and Mixtures Worksheet Answers
- Atoms Elements and Compounds Worksheets
- Elements Compounds and Mixtures Worksheet Answers
- Atoms Elements and Compounds Worksheets
- Elements Compounds and Mixtures Worksheet Answers
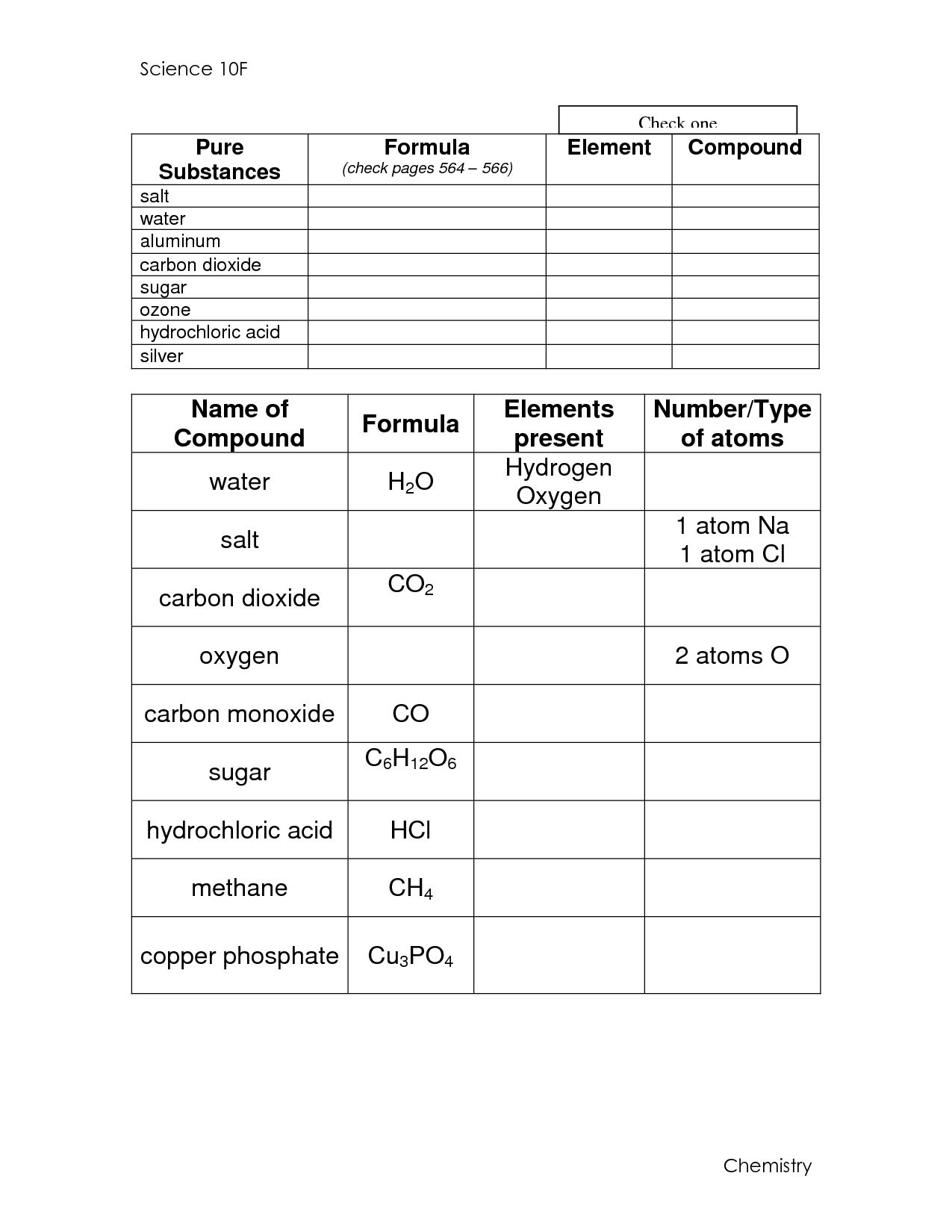
- Elements Compounds and Mixtures Answer Key
- Pure Substances and Mixtures Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
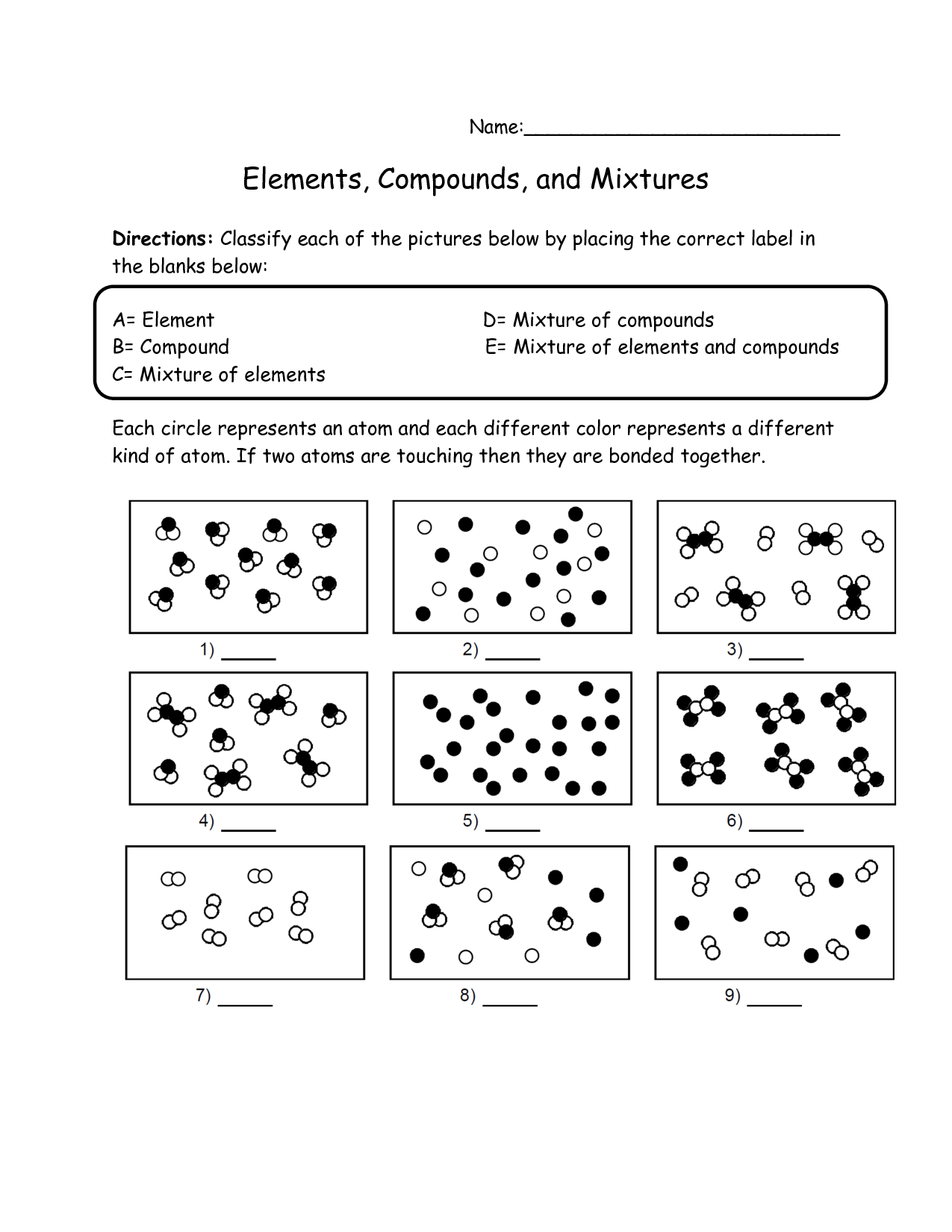
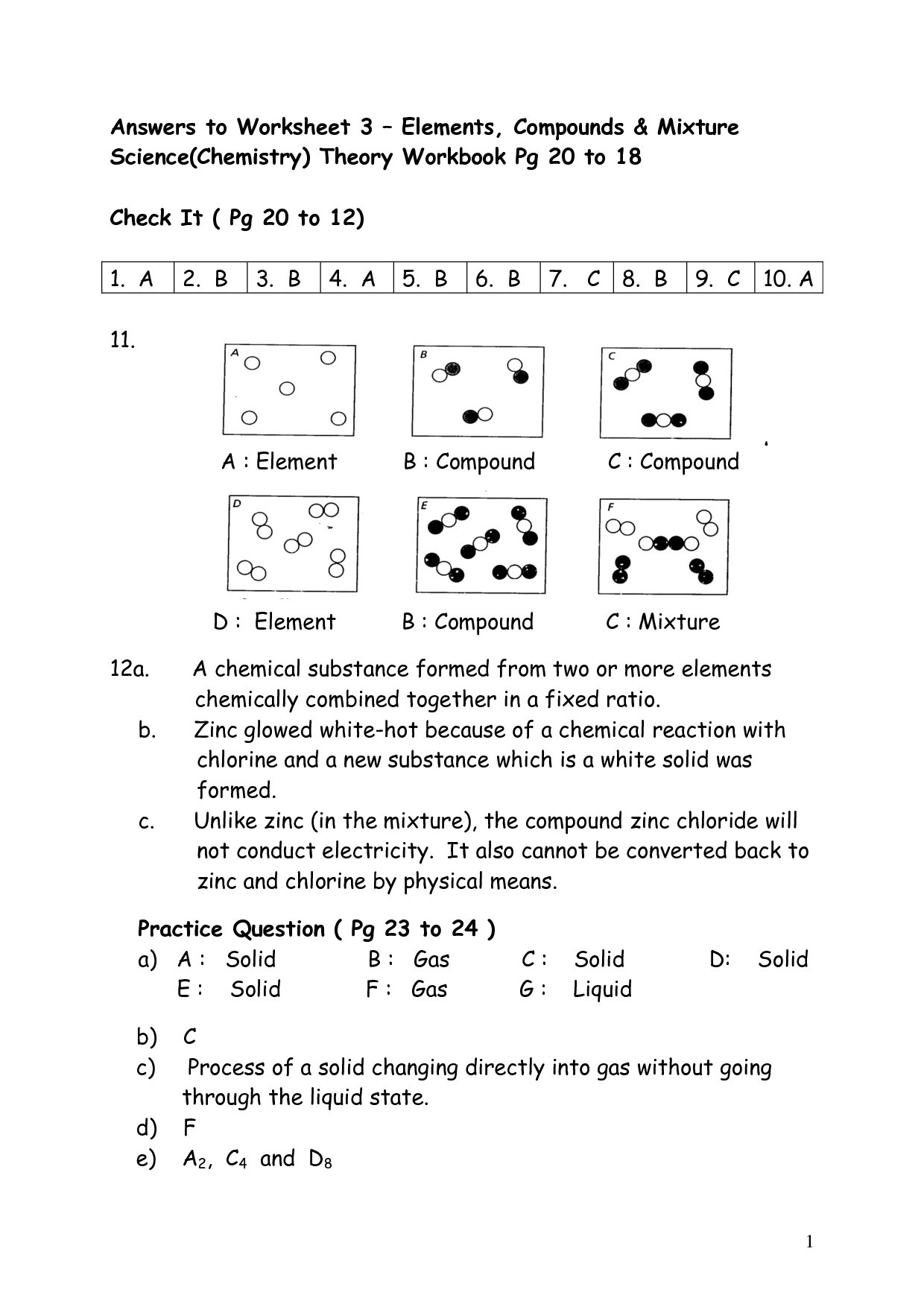
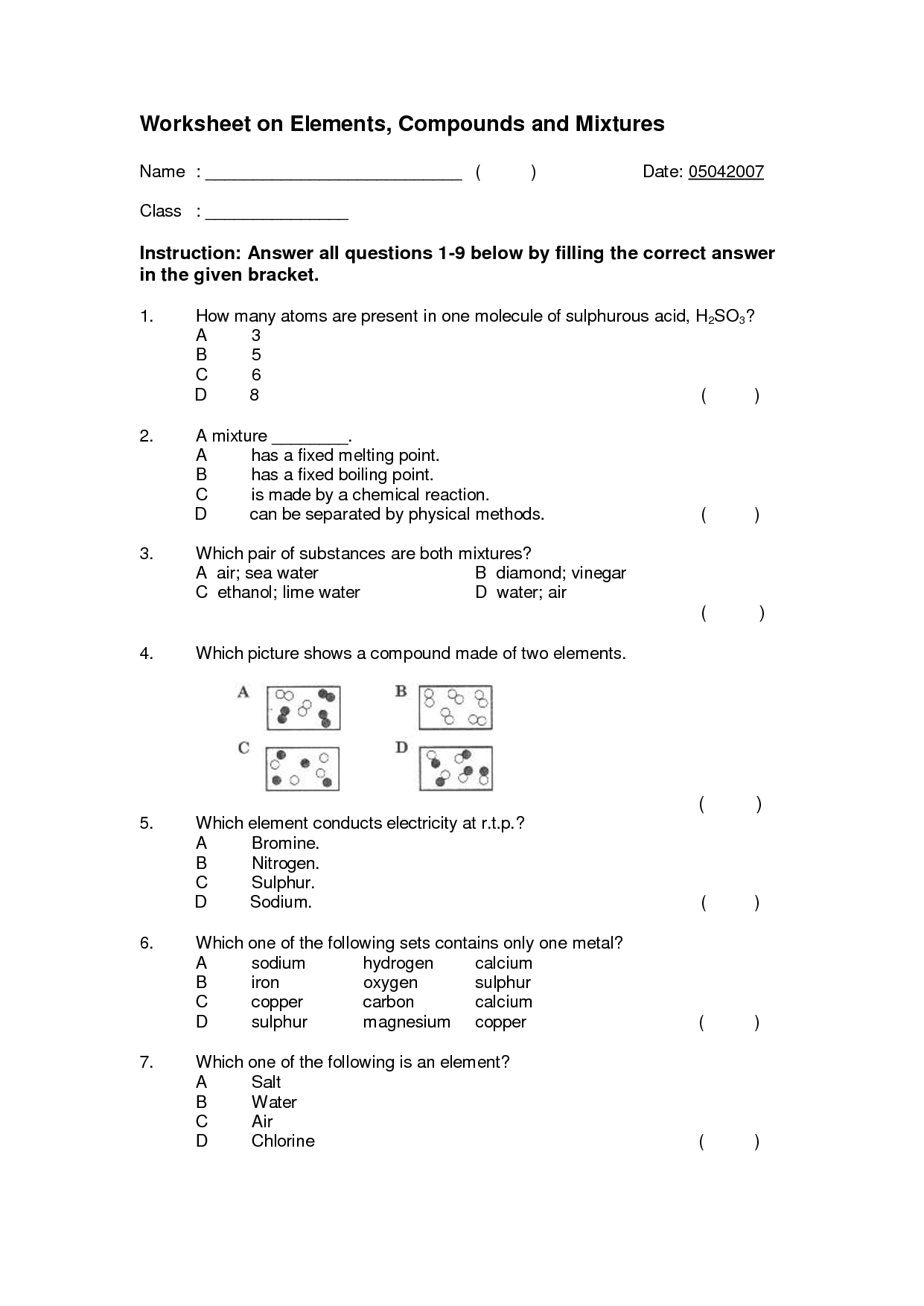
What is an element?
An element is a substance that consists of only one type of atom. It is the simplest form of matter that cannot be broken down into simpler substances by chemical means. Elements are represented by symbols and organized in the periodic table based on their atomic number and properties.
Give an example of an element.
One example of an element is oxygen, which has the chemical symbol O and atomic number 8. Oxygen is a key element essential for life, found in the earth's atmosphere and in many compounds such as water and carbon dioxide.
What is a compound?
A compound is a substance formed by the chemical combination of two or more different elements in fixed proportions. These elements are held together by chemical bonds, resulting in a new substance with unique physical and chemical properties distinct from the individual elements composing it.
Provide an example of a compound.
Table salt (sodium chloride) is an example of a compound. It is made up of sodium ions and chloride ions bonded together in a specific ratio, forming a distinct substance with unique properties separate from its individual elements.
How are elements and compounds different?
Elements are pure substances made up of only one type of atom, such as oxygen or gold. Compounds, on the other hand, are composed of two or more different elements chemically bonded together in a fixed ratio, forming a new substance with unique properties.
What is a mixture?
A mixture is a combination of two or more substances in which each substance retains its individual chemical properties. It can be a physical combination of solids, liquids, or gases, and it can be separated into its original components by physical means such as filtration, distillation, or evaporation.
Give an example of a mixture.
Trail mix is an example of a mixture, as it consists of a combination of different ingredients such as nuts, seeds, dried fruits, and chocolates blended together. Each individual component maintains its original properties and can be easily separated from the overall mixture.
Can mixtures be separated into their components? Give an example.
Yes, mixtures can be separated into their components through various methods such as filtration, distillation, chromatography, and evaporation. For example, a mixture of salt and water can be separated by evaporating the water and leaving behind the salt as a solid residue.
How are mixtures different from compounds?
Mixtures are combinations of two or more substances that are physically mixed together, such as salt and sugar, where the substances retain their individual properties and can be separated easily. Compounds, on the other hand, are substances formed by chemical bonds between different elements, resulting in a new substance with unique properties different from those of the individual elements, such as water (H2O). These compounds cannot be easily separated into their individual components without breaking the chemical bonds.
Provide an example of a substance that can exist as an element, a compound, and a mixture.
One example of a substance that can exist as an element, a compound, and a mixture is carbon. Carbon can exist as an element in its pure form as graphite or diamond, as a compound in carbon dioxide, and in various mixtures such as coal, wood, and organic matter in living organisms.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



























Comments