Electron Configuration Practice Worksheet
Are you a high school or college student studying chemistry? Do you find it difficult to grasp the concept of electron configuration? Look no further! This electron configuration practice worksheet is designed to help you reinforce your understanding of this fundamental topic.
Table of Images 👆
- Electron Configuration Worksheet Answers
- Electron Configuration Worksheet and Answers
- Electron Configuration and Lots More Worksheet Answers
- Electron Configuration Worksheet
- Electron Configuration and Quantum Numbers Worksheet
- Electron Configuration Worksheet
- Electron Shell Atom Diagram Worksheet
- Chemistry Stoichiometry Worksheet Answer Key
- Electron Configuration Worksheet Answer Key
- Naming Ionic Compounds Worksheet Answer Key
- Naming Ionic Compounds Worksheet Answers
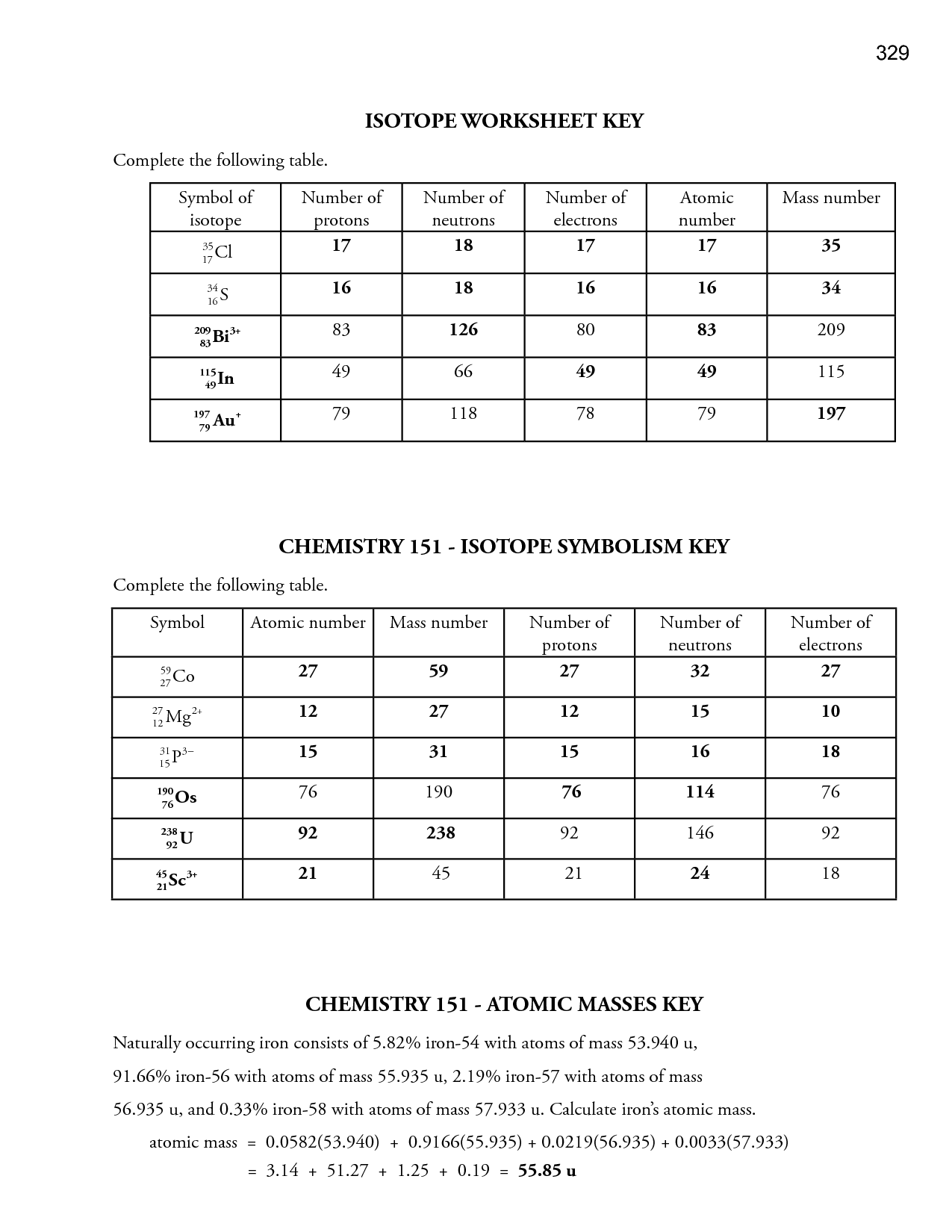
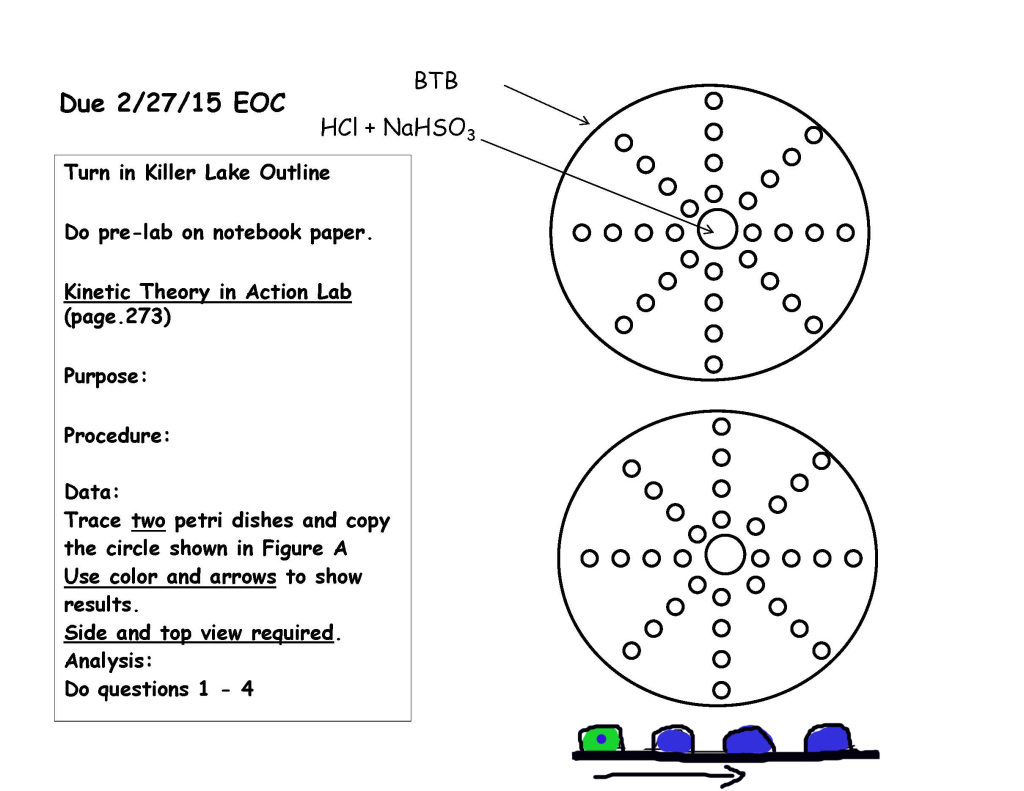
- Isotopes Worksheet Answer Key
- Specific Heat Thermochemistry Practice Problems Answer Key
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is electron configuration?
Electron configuration is a way of representing the arrangement of electrons in an atom's energy levels or shells. It describes the distribution of electrons among the orbitals of an atom in terms of the energy levels and sublevels they occupy. The electron configuration of an atom can provide information about its chemical properties and behavior in reactions.
How are electron configurations written?
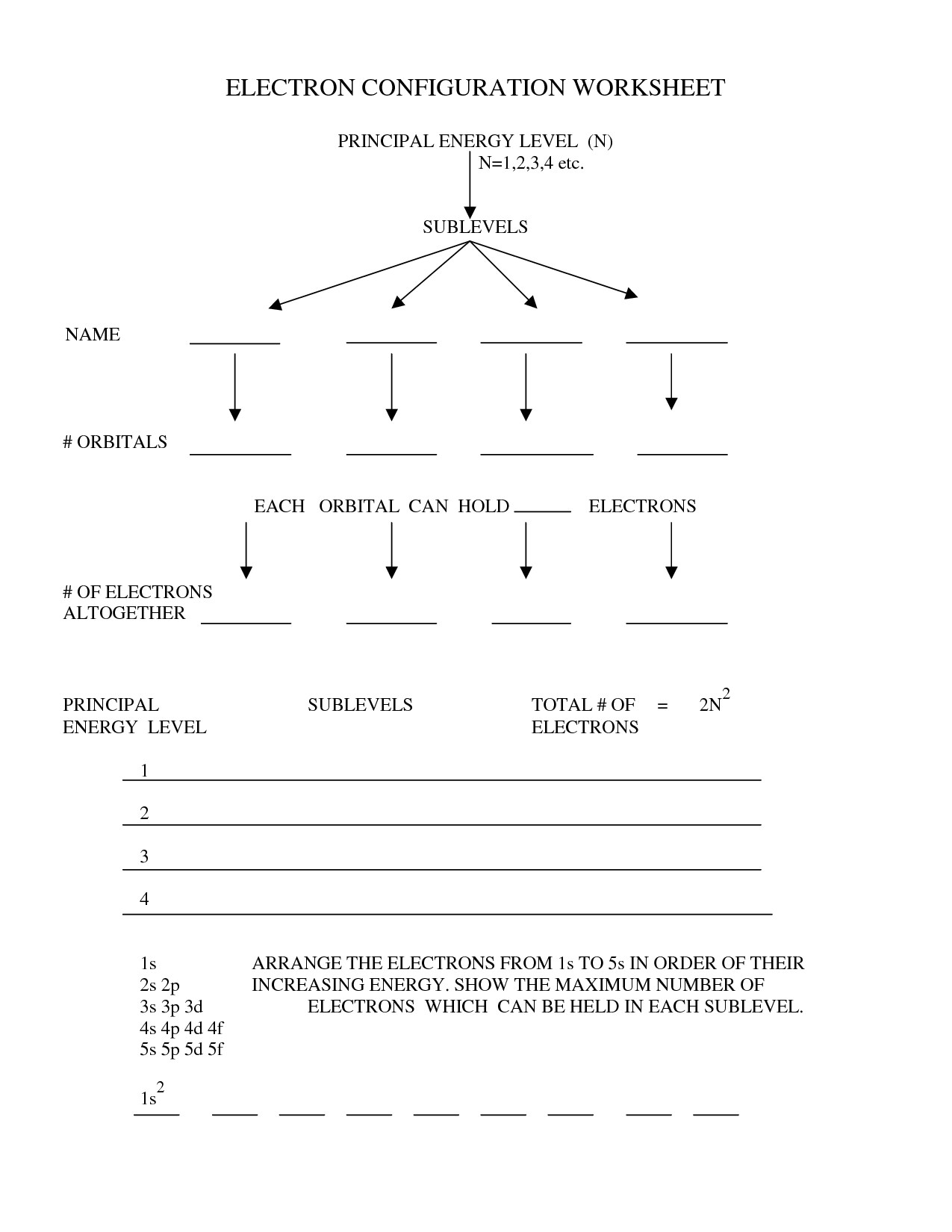
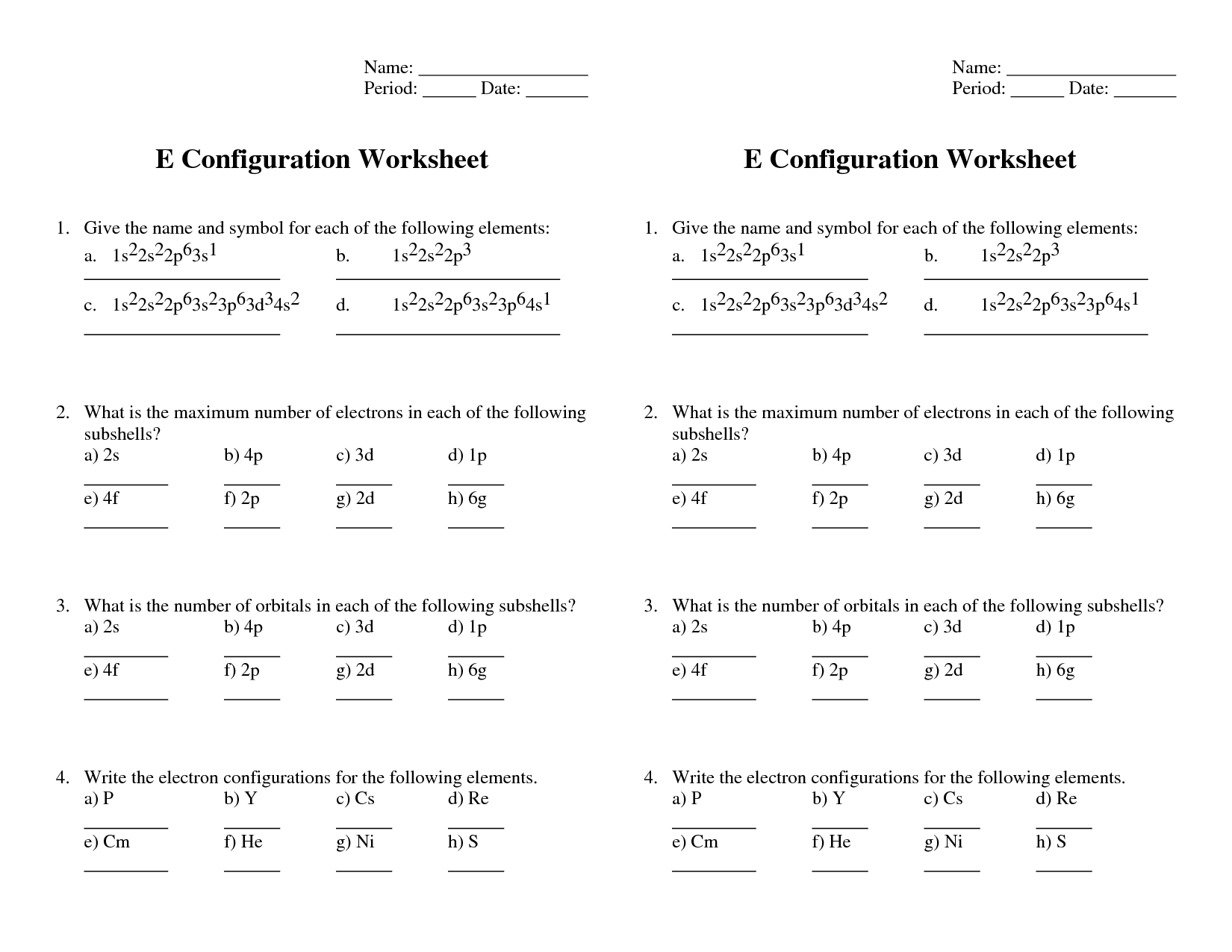
Electron configurations are written by listing the sublevels in ascending order of energy and indicating the number of electrons in each sublevel with a superscript. For example, the electron configuration of carbon would be written as 1s^2 2s^2 2p^2, showing that carbon has 6 electrons occupying the 1s, 2s, and 2p orbitals.
What is the purpose of electron configuration practice?
Practicing electron configurations helps students understand how electrons are organized within atoms and how they affect an element's properties. It enables them to predict chemical behavior, bonding patterns, and the reactivity of elements. Additionally, mastering electron configurations is crucial for understanding the periodic table and how elements are arranged based on their electron structures.
How does electron configuration help predict an atom's properties?
Electron configuration helps predict an atom's properties because it determines how the electrons are arranged in an atom's energy levels, which in turn influences its reactivity, bonding behavior, and chemical properties. By understanding the distribution of electrons among different energy levels and sublevels, scientists can predict an atom's stability, ability to form bonds, and its overall behavior in chemical reactions. The number and arrangement of electrons in an atom's orbitals play a crucial role in determining its physical and chemical characteristics, making electron configuration a key factor in predicting an atom's properties.
How many electrons can each orbital hold?
The s orbital can hold a maximum of 2 electrons, the p orbital can hold a maximum of 6 electrons, the d orbital can hold a maximum of 10 electrons, and the f orbital can hold a maximum of 14 electrons.
How are electrons distributed among different energy levels?
Electrons are distributed among different energy levels in an atom according to the principles of the Aufbau principle, Pauli exclusion principle, and Hund's rule. The Aufbau principle states that electrons fill the lowest energy levels first before moving to higher ones, while the Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. Hund's rule further dictates that electrons will occupy orbitals singly before pairing up. This results in the distribution of electrons in the atom's energy levels in a way that minimizes the overall energy of the system.
What are the rules for filling orbitals in electron configuration?
The rules for filling orbitals in electron configuration are based on the Aufbau principle, Hund's rule, and the Pauli exclusion principle. The Aufbau principle dictates that electrons fill orbitals starting from the lowest energy level to the highest. Hund's rule states that electrons fill orbitals singly before pairing up, maximizing the spins within a subshell. The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers, meaning that each orbital can hold a maximum of two electrons with opposite spins.
What is the significance of the noble gas notation in electron configuration?
The noble gas notation in electron configuration is significant because it allows us to represent the electron configuration of an element more efficiently by using the electron configuration of a noble gas that precedes it in the periodic table. This notation simplifies the representation of complex electron configurations, making it easier to understand and predict the chemical behavior of elements. Additionally, it clearly shows the outermost electron shell of an atom, which is crucial in determining its reactivity and bonding patterns.
How does electron configuration vary across the periodic table?
Electron configuration varies across the periodic table due to the arrangement of elements and their atomic number. In general, as you move from left to right across a period, the number of electrons in the outermost energy level increases by one with each element, resulting in a similar pattern in electron configuration. Moving down a group, the principal energy level increases, leading to a change in electron configuration due to the added energy levels. This pattern is a result of the positioning of elements in the periodic table based on their atomic structure.
How can electron configuration be used to determine the reactivity of elements?
Electron configuration can be used to determine the reactivity of elements by indicating the number of valence electrons an atom has and how readily it can gain, lose, or share electrons to achieve a stable electron configuration. Elements with a few valence electrons are more likely to lose them to achieve a stable octet and become positively charged cations, increasing reactivity. On the other hand, elements with almost a full valence shell are more likely to gain electrons to achieve a stable octet and become negatively charged anions, also increasing reactivity. Therefore, elements with similar electron configurations tend to exhibit similar reactivity patterns.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.
























Comments