Egg and Vinegar Experiment Worksheet
The Egg and Vinegar Experiment Worksheet is a valuable tool for educators and parents looking to engage their students in a hands-on science activity. This worksheet is designed to guide learners through the process of conducting the egg and vinegar experiment, while highlighting key points, observations, and results. With a focus on exploring chemical reactions and the concept of acidity, this worksheet suits curious young scientists eager to unravel the mysteries of the natural world.
Table of Images 👆
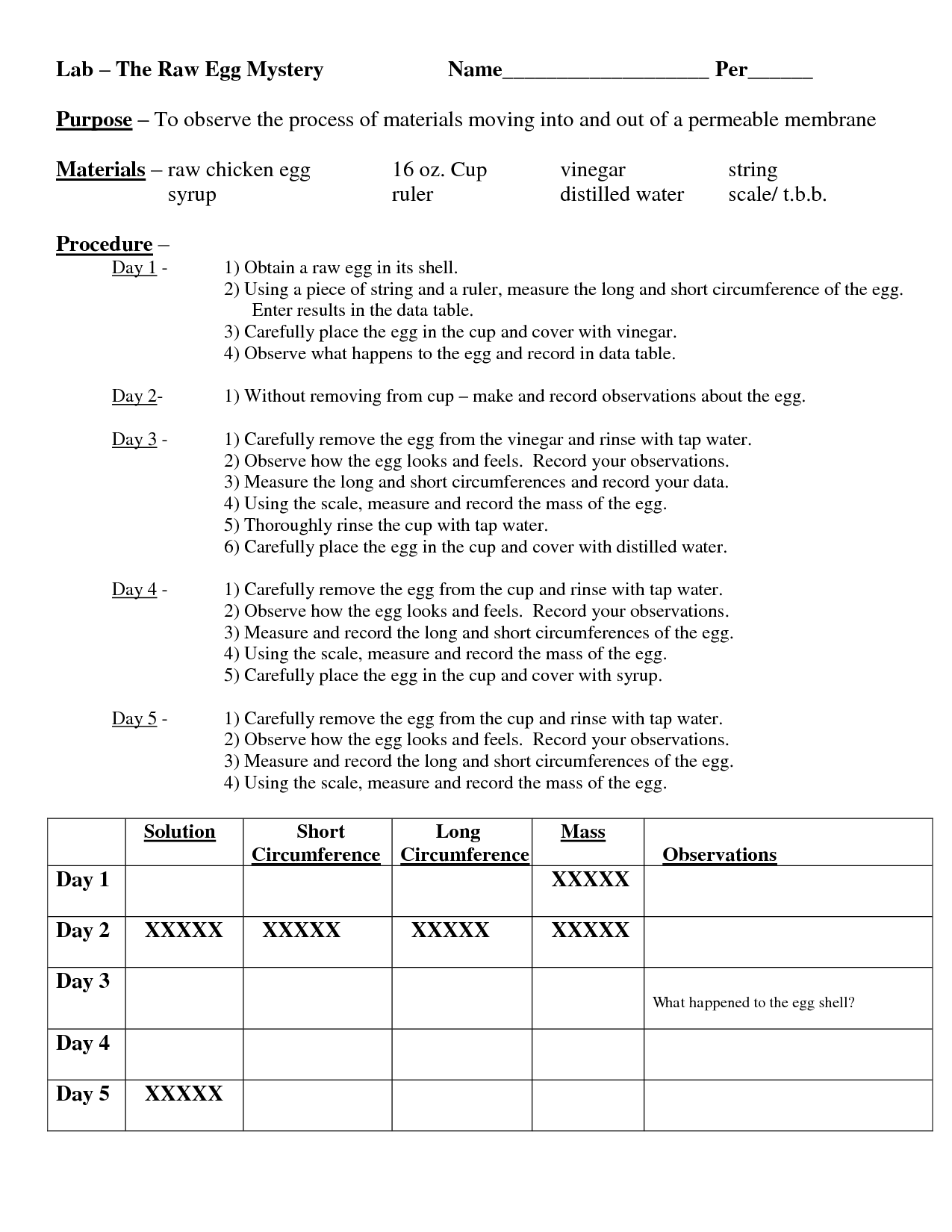
- Osmosis and Diffusion Egg Lab
- Vinegar Egg Lab Worksheet
- Diffusion and Osmosis Worksheet Answers
- Egg Experiment Worksheets
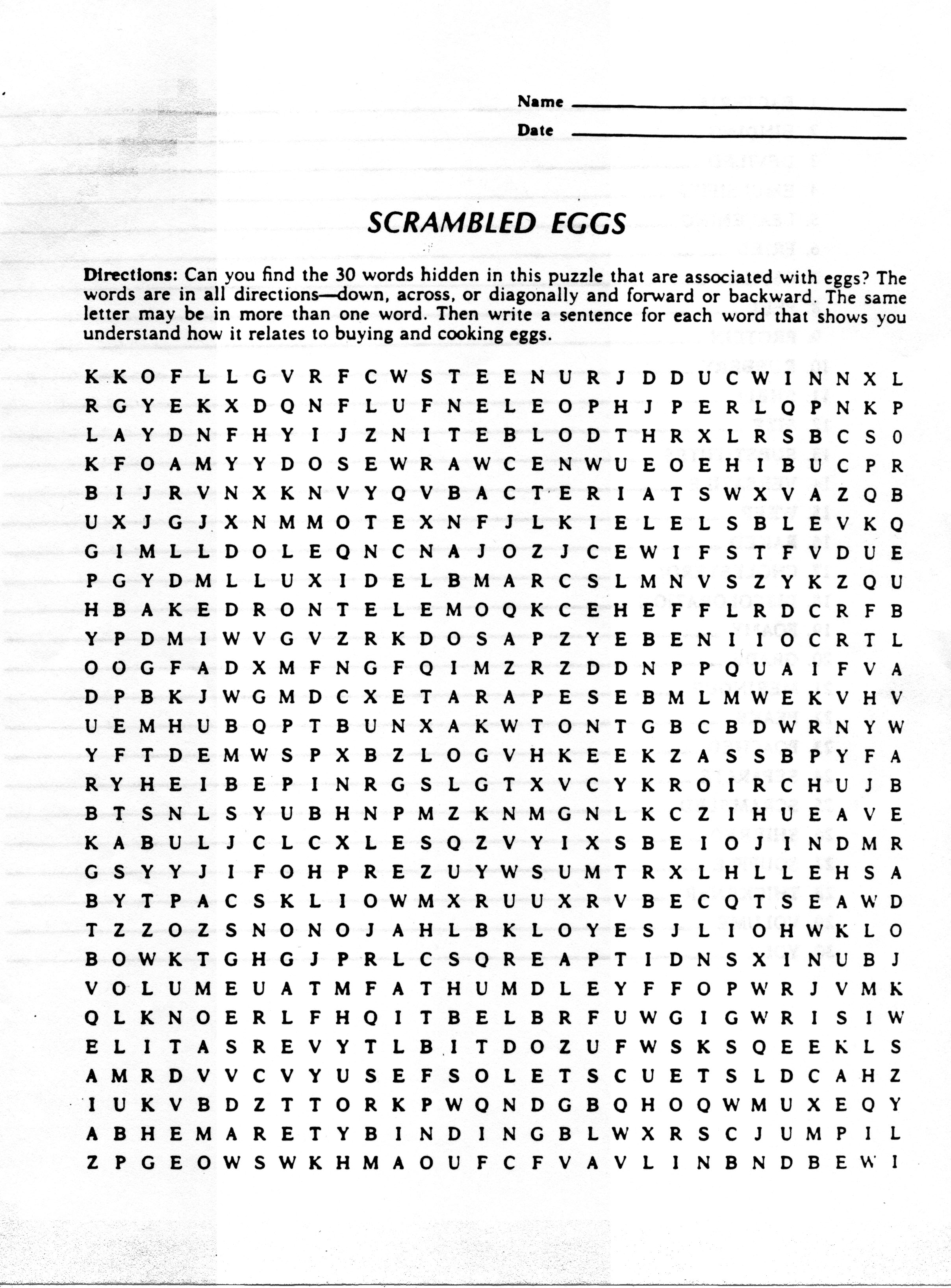
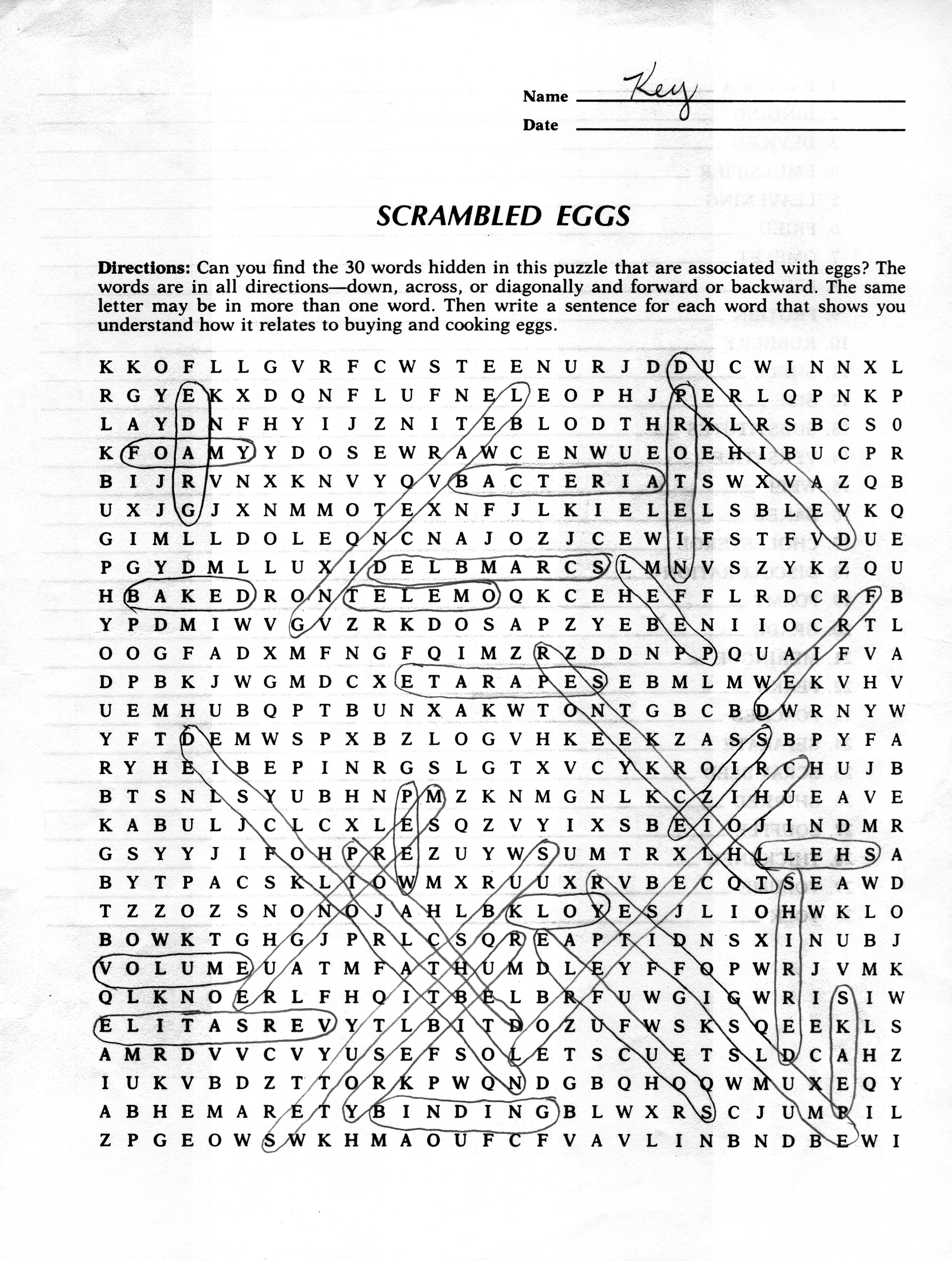
- Scrambled Eggs Worksheet Key
- Egg with Stain Teeth Science Experiment Worksheets
- Egg Nutrition Worksheets High School
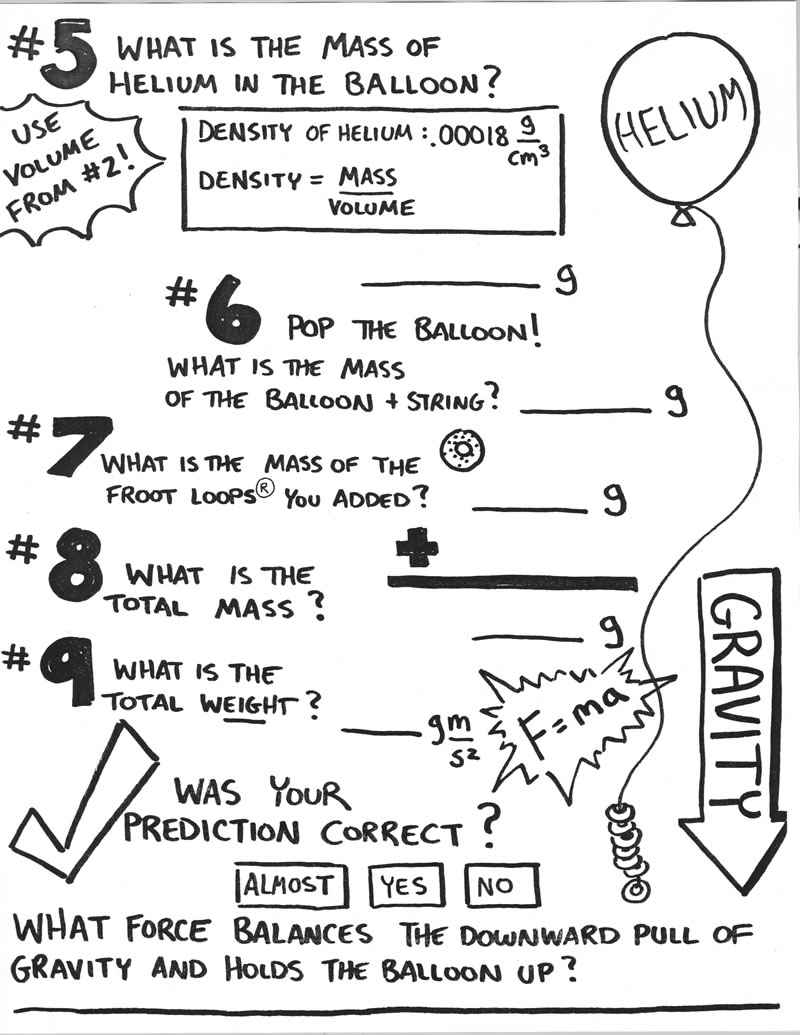
- Balloon Rocket Experiment Worksheet
- Egg Osmosis Hypertonic Hypotonic Isotonic Solution
- Science Experiment Worksheet Scientific Method
- Science Experiment Worksheet
- 5th Grade Science Study Worksheets
- Biome Classification Worksheet
- Egg Osmosis Lab Conclusion
- Egg Osmosis Lab Report Results
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is the purpose of the egg and vinegar experiment?
The purpose of the egg and vinegar experiment is to demonstrate the process of osmosis. When an egg is placed in vinegar, the vinegar breaks down the calcium carbonate in the eggshell, leaving behind a semipermeable membrane. This membrane allows water to move in and out of the egg, causing it to swell as water enters through the process of osmosis. Through this experiment, one can observe how osmosis works and understand the concept of membrane permeability.
What happens when the egg is placed in vinegar?
When an egg is placed in vinegar, the shell of the egg dissolves because of the acidic nature of the vinegar. The vinegar breaks down the calcium carbonate in the shell, leaving behind the egg's membrane, which gives it a rubbery texture and allows it to bounce. This experiment demonstrates how acids can break down certain materials.
How does the eggshell change over time?
The eggshell undergoes changes over time as the egg ages. As it gets older, the eggshell can become more porous and thinner, making it more susceptible to breakage. The color of the eggshell may also lighten or darken as the egg ages. Additionally, older eggs may lose some of their protective coating, making them more vulnerable to bacteria and spoilage.
What causes the bubbling reaction between the vinegar and eggshell?
The bubbling reaction between vinegar and eggshell is caused by a chemical reaction known as an acid-base reaction. Vinegar, which is acidic due to the presence of acetic acid, reacts with the calcium carbonate in the eggshell, which is a base. This reaction produces carbon dioxide gas, which forms bubbles you see on the surface of the eggshell. The reaction also breaks down the calcium carbonate into calcium acetate, water, and carbon dioxide.
What is the chemical reaction that occurs during the experiment?
The chemical reaction that occurs during the experiment involves the oxidation of magnesium metal to form magnesium oxide. This reaction can be represented by the following equation: 2Mg(s) + O2(g) -> 2MgO(s), where magnesium (Mg) reacts with oxygen (O2) in the air to produce magnesium oxide (MgO).
How does the egg's appearance change after being soaked in vinegar?
After being soaked in vinegar, the egg's appearance changes as the acidic vinegar dissolves the eggshell, revealing the translucent membrane of the egg underneath. The eggshell becomes porous and begins to bubble and fizz as it reacts with the vinegar, gradually breaking down and dissolving until only the inner membrane is left intact.
Why does the egg become soft and squishy?
The softness and squishiness of an egg can occur due to the breakdown of its structure and properties. This deterioration can happen through various factors such as age, temperature, and exposure to air. As the proteins in the egg start to break down and water evaporates, the egg loses its firmness and becomes soft and squishy. Additionally, bacterial contamination or physical damage can also contribute to the softening of an egg.
What happens if the egg is left in vinegar for an extended period?
If an egg is left in vinegar for an extended period, the vinegar will dissolve the eggshell due to its acidic nature. Over time, the eggshell will break down and the egg will be left with only the membrane encasing the egg yolk and white.
Can you describe the texture of the egg after its shell is dissolved?
Once the eggshell is dissolved, the egg's texture becomes softer and more delicate, resembling a semi-solid gel-like substance. The egg white and yolk mix together, resulting in a smooth and creamy texture that is uniform throughout. It is no longer contained within its shell, making it easier to mix or incorporate into recipes.
What can be learned from conducting the egg and vinegar experiment?
The egg and vinegar experiment can teach us about the principles of osmosis. When an egg is placed in vinegar, the acidic solution breaks down the calcium carbonate shell, leaving the egg membrane intact. Over time, the vinegar penetrates the egg through osmosis, causing it to swell as water moves inside. This process demonstrates how substances can move across a semipermeable membrane in response to concentration gradients, a fundamental concept in biology and chemistry.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments