Density Practice Worksheet Middle School
Are you a middle school student looking to enhance your understanding of density? If so, you've come to the right place! In this blog post, we will explore the value of worksheets as a valuable tool for practicing and mastering the concept of density.
Table of Images 👆
- Density Worksheet Middle School
- 6th-Grade Calculating Density Worksheet
- Density Calculations Worksheet
- Chemistry Density Worksheet Answers
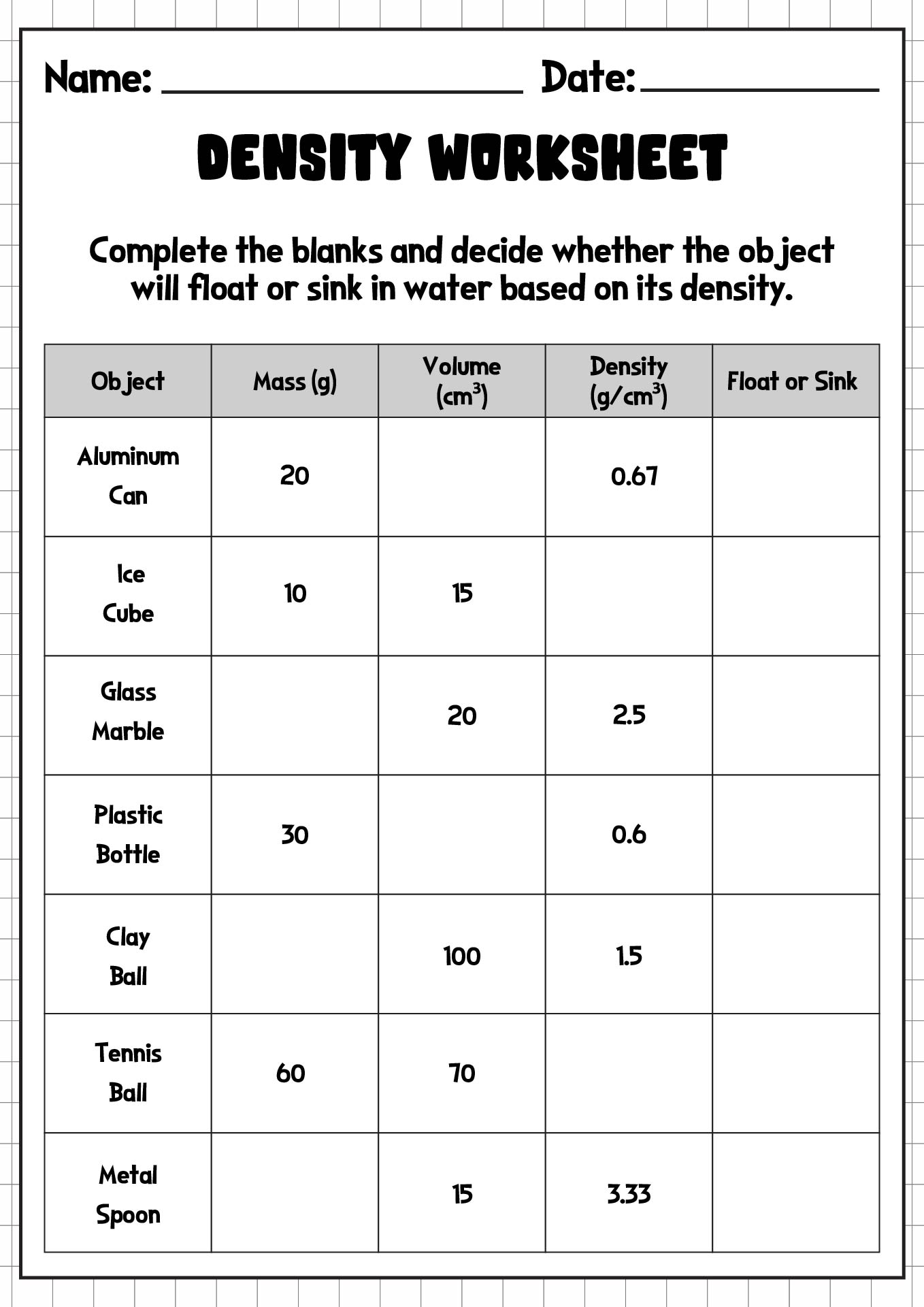
- Density Worksheet
- Density Column Worksheet
- Density Practice Worksheet
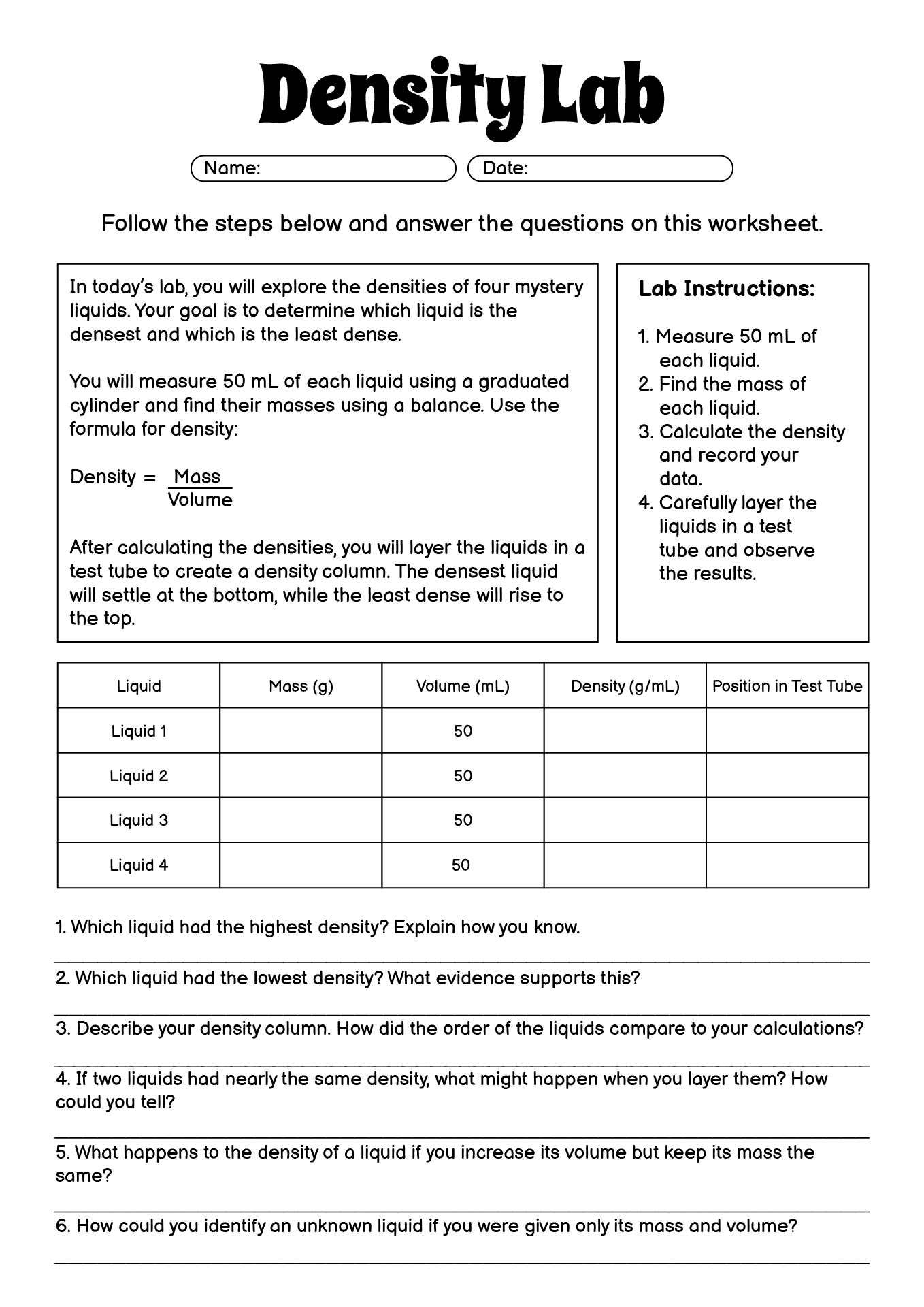
- Density Lab Middle School Worksheets
- Middle School Density Lab Worksheet
- Science Worksheet on Density for Middle Schoolers
- Density Calculation Exercises for Middle School Students

More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is density?
Density is a measure of how much mass is contained in a given volume of a substance. It is calculated by dividing the mass of an object by its volume. Materials with higher density have more mass packed into a smaller volume, while materials with lower density have less mass in a larger volume. Density is often expressed in units such as grams per cubic centimeter or kilograms per liter.
How is density calculated?
Density is calculated by dividing the mass of an object by its volume. The formula for density is: Density = Mass / Volume. Mass is typically measured in grams or kilograms, while volume is usually measured in milliliters, cubic centimeters, or liters. Density is expressed in units such as grams per cubic centimeter (g/cm^3) or kilograms per liter (kg/L).
What is the unit of measurement for density?
The unit of measurement for density is typically expressed in kilograms per cubic meter (kg/m^3) in the metric system or in grams per cubic centimeter (g/cm^3) in the CGS (centimeter-gram-second) system.
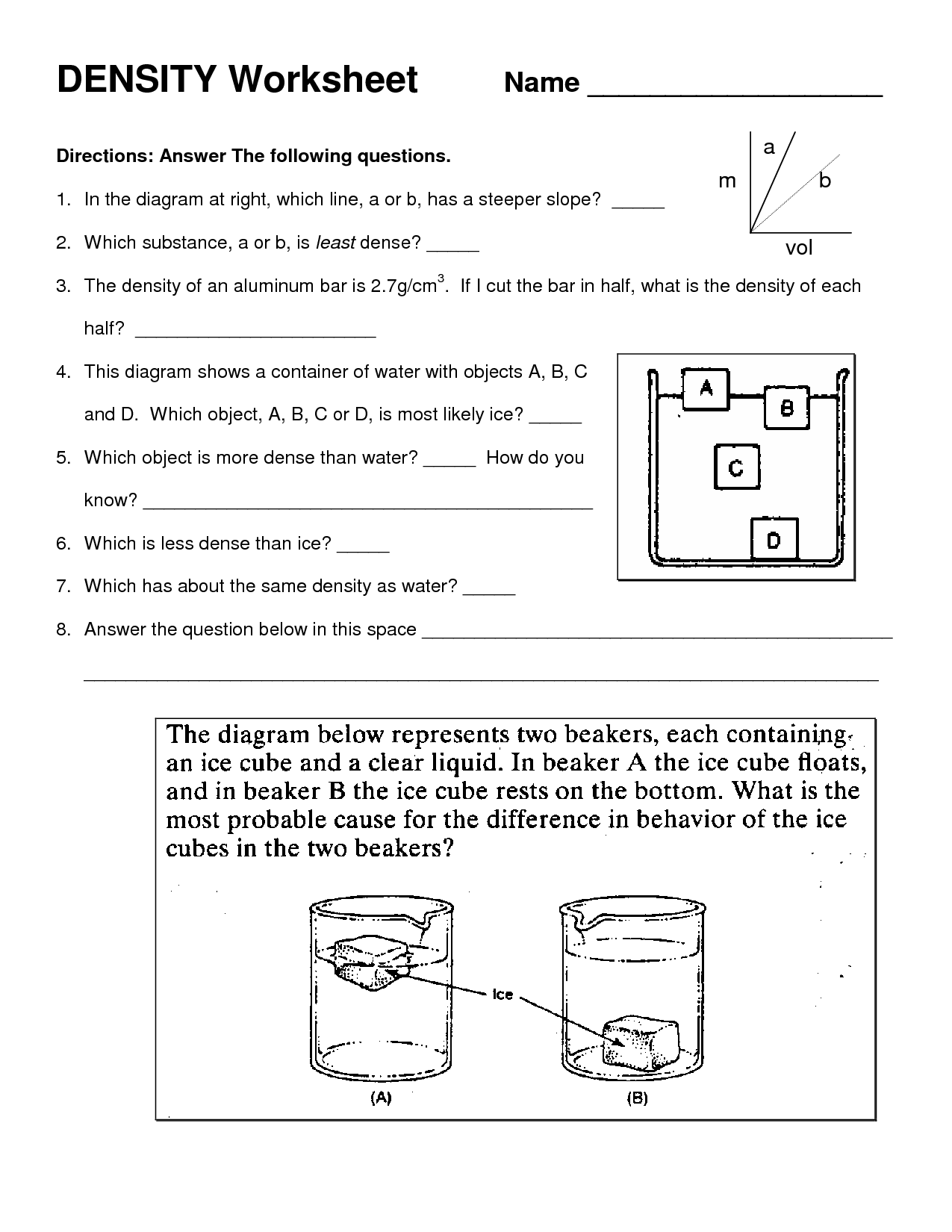
How does the density of a substance determine whether it sinks or floats in a liquid?
The density of a substance determines whether it sinks or floats in a liquid because an object will float if it is less dense than the liquid it is placed in and will sink if it is more dense. In other words, if the density of the substance is higher than the density of the liquid, it will sink, and if it is lower, it will float. This is due to the principle of buoyancy, where the upward force exerted by the liquid on the object (buoyant force) is greater than the force of gravity pulling the object downward if the object is less dense than the liquid, causing it to float.
How does the density of an object affect its ability to float in water?
The density of an object affects its ability to float in water because if the object's density is less than that of water, it will float. This is due to the principle of buoyancy, where the upward force exerted by the water on the object is greater than the downward force exerted by the object's weight. Objects with lower density displace more water and experience a greater upward force, allowing them to float. Conversely, objects with higher density will sink in water as the downward force is greater than the upward buoyant force.
What is the relationship between mass, volume, and density?
Density is the ratio of an object's mass to its volume. Mathematically, density = mass/volume. Mass is the amount of matter in an object, while volume is the amount of space it occupies. Therefore, the relationship between mass, volume, and density is that density is determined by the mass of an object relative to its volume - as the mass of an object increases, its density also increases if the volume remains constant, and vice versa.
What factors can cause the density of a substance to change?
The density of a substance can change due to various factors such as temperature, pressure, and composition. For example, as temperature increases, the particles in a substance move faster and farther apart, leading to a decrease in density. On the other hand, an increase in pressure can cause the particles to pack closer together, resulting in a higher density. Additionally, changes in the composition of a substance, such as through mixing or chemical reactions, can also alter its density.
How does temperature affect the density of a substance?
Temperature affects the density of a substance by changing the spacing and movement of its particles. As temperature increases, the particles move more rapidly and spread out, causing the substance to expand and decrease in density. Conversely, as temperature decreases, particles move slower and become more compact, leading to an increase in density.
How does the density of a gas compare to the density of a liquid or solid?
The density of a gas is generally much lower than the density of a liquid or solid. This is because the molecules in a gas are more spread out and have higher kinetic energy, leading to a lower overall mass in a specific volume compared to a liquid or solid where the molecules are more closely packed together.
How is density used in everyday life or in various scientific fields?
Density is a crucial concept used in both everyday life and various scientific fields. In everyday life, density is utilized in cooking to adjust the texture of food, in construction to determine the strength of materials, and in the automotive industry to optimize fuel efficiency. In scientific fields like geology, chemistry, and physics, density plays a significant role in identifying materials, determining the composition of substances, and predicting the behavior of fluids. Additionally, in environmental science, density measurements aid in studying air and water pollution levels.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.


























Comments