Classifying Chemical Reactions Worksheet Answers
If you're a student studying chemistry, having access to accurate and reliable resources is key to success. One essential tool that can assist you in understanding and classifying chemical reactions is a worksheet with detailed and comprehensive answers.
Table of Images 👆
- Reaction Types Worksheet Answer Key
- Practice Balancing Chemical Equations Worksheet
- Types of Chemical Reactions Worksheet Answers
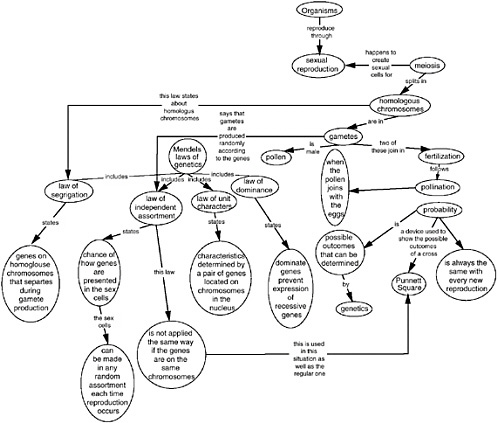
- Meiosis Terminology Concept Map Answers
- Chemistry Equations Answer Key Chapter 10 Review
- Balancing Chemical Equations Worksheet Answer Key
- Classification of Matter Worksheet Answers
- Chapter 11 Introduction to Genetics Worksheet
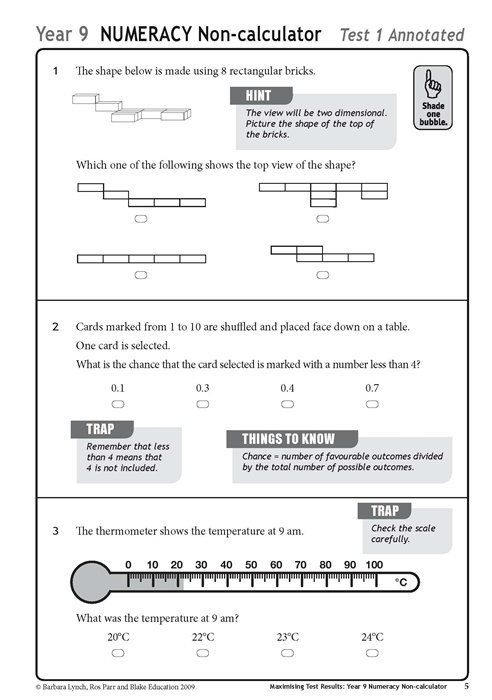
- Test Formats for Teachers
- Test Formats for Teachers
- Test Formats for Teachers
- Test Formats for Teachers
- Test Formats for Teachers
- Test Formats for Teachers
- Test Formats for Teachers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is a chemical reaction?
A chemical reaction is a process where one or more substances are transformed into new substances by breaking and forming chemical bonds. It involves the rearrangement of atoms to create different molecules with different properties than the original substances.
What are the signs that a chemical reaction has occurred?
Some signs that a chemical reaction has occurred include the formation of a precipitate (solid substance formed from a liquid solution), a color change in the reactants or products, the evolution of gas bubbles, a change in temperature (exothermic or endothermic reactions), and the production of light or sound. These observable changes indicate that new substances with different properties have been formed due to the rearrangement of atoms during the chemical reaction.
What is a synthesis reaction?
A synthesis reaction is a type of chemical reaction where two or more reactants combine to form a single product. This reaction typically involves the creation of a more complex or larger molecule from simpler reactants. In a synthesis reaction, the atoms and bonds in the reactants are rearranged to form the product molecule.
What is a decomposition reaction?
A decomposition reaction is a type of chemical reaction where a single compound breaks down into two or more simpler substances. This process can be triggered by heat, light, electricity, or addition of a catalyst.
What is a combustion reaction?
A combustion reaction is a chemical reaction in which a substance combines rapidly with oxygen to produce heat and light, typically in the form of a flame. This reaction usually involves a fuel, such as hydrocarbons, combining with oxygen gas to form carbon dioxide, water, and heat energy as products.
What is a single replacement reaction?
A single replacement reaction is a type of chemical reaction in which an element replaces another element in a compound, resulting in the formation of a new compound and a different element. This reaction occurs when a more reactive element displaces a less reactive element in a compound.
What is a double replacement reaction?
A double replacement reaction is a chemical reaction where two compounds exchange ions to form two new compounds. In this type of reaction, the cations and anions of the reacting compounds switch partners, resulting in the formation of two different compounds. Double replacement reactions typically occur in aqueous solutions and are also known as double displacement reactions.
What is an acid-base reaction?
An acid-base reaction is a chemical reaction that occurs between an acid and a base, resulting in the formation of water and a salt. Acids are substances that release hydrogen ions (H+) in solution, while bases are substances that release hydroxide ions (OH-) in solution. When an acid and a base react, the H+ ions from the acid combine with the OH- ions from the base to form water. The remaining ions then combine to form a salt. This reaction is important in neutralizing acidic or basic solutions, and plays a crucial role in various chemical and biological processes.
What is a redox reaction?
A redox reaction, short for reduction-oxidation reaction, is a type of chemical reaction that involves the transfer of electrons between substances. Reduction refers to the gain of electrons by a molecule, atom, or ion, while oxidation refers to the loss of electrons. In redox reactions, one substance is oxidized (loses electrons) while another is reduced (gains electrons), with the overall result being a transfer of electrons from one reactant to another.
What are the different ways to classify chemical reactions?
Chemical reactions can be classified based on various criteria, such as the type of reactants involved (combination, decomposition, displacement, double displacement), the energy changes that occur during the reaction (exothermic, endothermic), the presence of catalysts, and the overall chemical composition of the reactants and products. Other classifications include oxidation-reduction reactions, acid-base reactions, and organic reactions based on the types of compounds involved and the nature of bond formation or breakage during the reaction.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments