Chemistry Unit 8 Worksheet 4
Are you a high school or college student studying chemistry? Look no further! We have a new resource that will help you reinforce your understanding of Unit 8 concepts. Introducing Chemistry Unit 8 Worksheet 4, designed to provide you with additional practice and engagement with the subject matter. This worksheet focuses specifically on Unit 8 topics, helping you solidify your knowledge and prepare for assessments.
Table of Images 👆
- Polar Bonds and Molecules Worksheet Review Answers Section
- Chemistry Unit 4 Worksheet 2 Answer Key
- Chemistry Unit Conversions Worksheet
- Chemistry pH and Poh Calculations Worksheet Answers
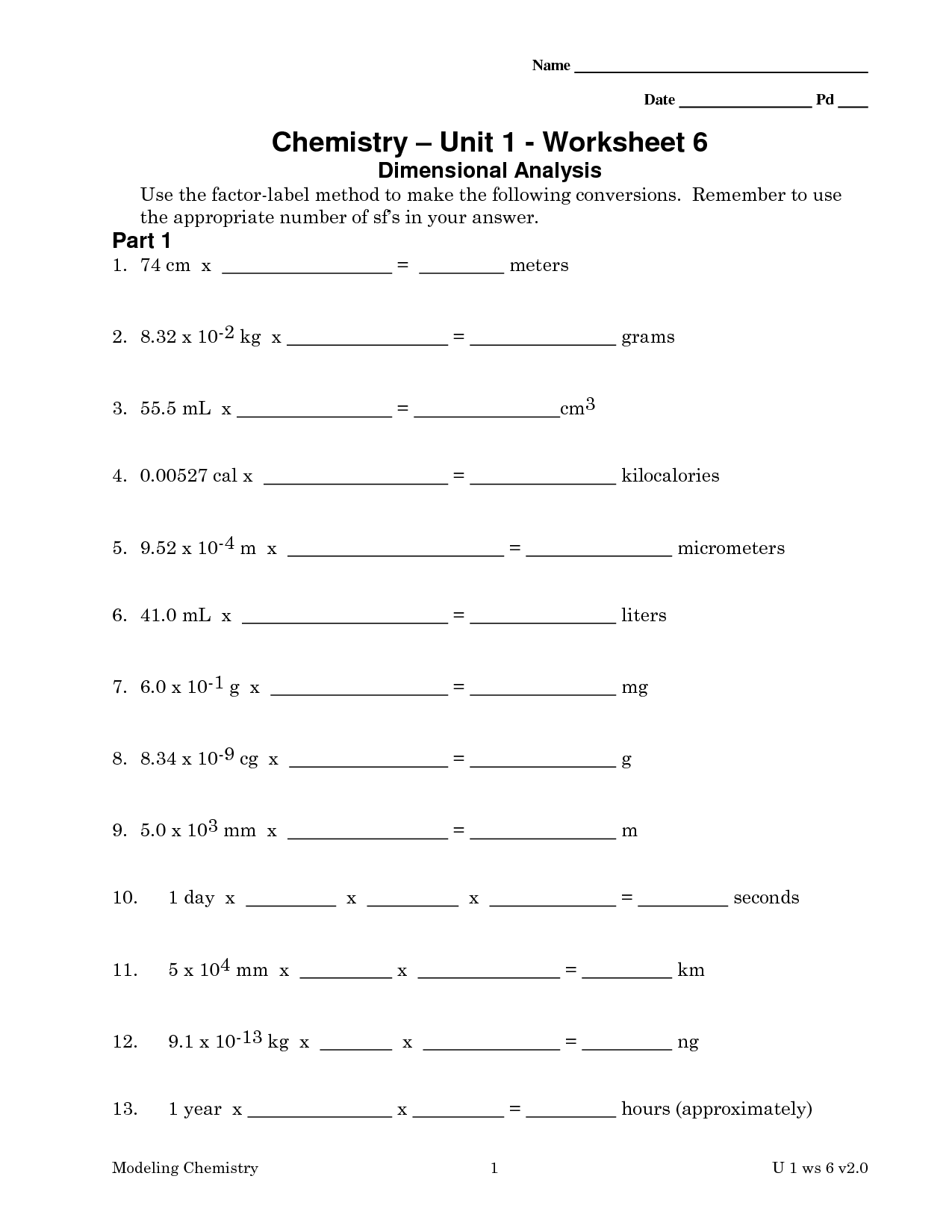
- Chemistry Unit 1 Worksheet 6
- Chemistry Unit 5 Worksheet 2 Answer Key
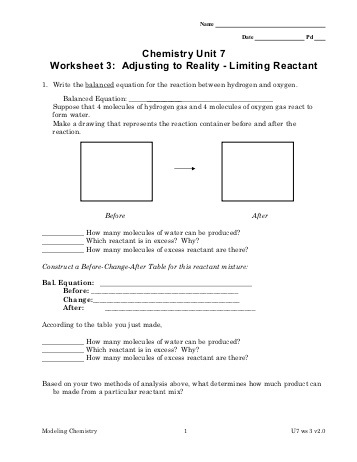
- Chemistry Unit 7 Worksheet 3
- Chemistry Stoichiometry Worksheet Answers
- Chemistry Worksheet Answer Keys
- Unit 3 Worksheet 4 Quantitative Energy Problems
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the purpose of balancing chemical equations?
The purpose of balancing chemical equations is to ensure that the law of conservation of mass is followed. This means that the number of each type of atom on the reactant side of the equation must be equal to the number of that same atom on the product side. Balancing the equation ensures that there is a correct understanding of the stoichiometry and amount of each substance involved in the reaction.
How does the Law of Conservation of Mass apply to chemical reactions?
The Law of Conservation of Mass states that in a chemical reaction, matter is neither created nor destroyed, only rearranged. This means that the total mass of the reactants must equal the total mass of the products. In other words, atoms are conserved in a chemical reaction, so the number and type of atoms on each side of the reaction equation must be the same. This principle is fundamental to understanding and predicting the outcomes of chemical reactions.
What are the key differences between reactants and products in a chemical equation?
Reactants are the starting materials in a chemical reaction that interact to produce new substances known as products. The key difference between reactants and products is that reactants are the original compounds or elements that undergo a chemical change, while products are the new substances formed as a result of the reaction. Reactants are written on the left side of a chemical equation, while products are written on the right side, reflecting the transformation that occurs during the reaction.
How do coefficients in a balanced chemical equation represent the ratio of moles?
Coefficients in a balanced chemical equation represent the ratio of moles by indicating the relative amounts of each reactant and product involved in the chemical reaction. The coefficients show the fixed mole-to-mole ratio in which substances react and are produced, allowing us to determine the exact quantities of substances consumed and formed in the reaction based on this ratio.
What role does a catalyst play in a chemical reaction?
A catalyst plays a crucial role in a chemical reaction by speeding up the rate of the reaction without being consumed itself. It lowers the activation energy required for the reaction to proceed, allowing the reaction to occur more quickly and efficiently. This increased reaction rate can result in cost savings, increased yield, and improved selectivity in the production of desired products.
What are the characteristics of a reversible reaction?
A reversible reaction is one that can proceed in both the forward and reverse directions, meaning that the products can react to reform the reactants. These reactions often reach a state of dynamic equilibrium where the forward and reverse reactions occur at the same rate. Reversible reactions are characterized by being affected by factors such as concentration, temperature, and pressure, which can shift the equilibrium towards either the products or reactants.
How does temperature affect the rate of a chemical reaction?
Temperature affects the rate of a chemical reaction by increasing the kinetic energy of molecules, leading to more frequent and energetic collisions between reactant molecules. This usually results in a higher probability of successful collisions and faster reaction rates. Additionally, higher temperatures can also lower the activation energy barrier for the reaction, allowing the reaction to proceed more easily and rapidly. Conversely, lower temperatures can slow down reaction rates as molecules move more slowly and collisions are less likely to result in a successful reaction.
What is the effect of a catalyst on the activation energy of a reaction?
A catalyst decreases the activation energy of a reaction by providing an alternative reaction pathway that has a lower activation energy barrier. This increase in reaction rate is accomplished by stabilizing the transition state, which allows the reaction to proceed more readily. In essence, a catalyst facilitates the formation of the transition state, leading to a lower overall activation energy and making the reaction occur more quickly.
Explain the concept of dynamic equilibrium in relation to chemical reactions.
Dynamic equilibrium in chemical reactions occurs when the rate of the forward reaction equals the rate of the reverse reaction, resulting in no net change in the concentrations of reactants and products over time. At this point, the system appears to be at rest, but in reality, the reactions are still occurring at equal rates. This state of equilibrium can be disrupted by changes in conditions such as temperature, pressure, or concentration, causing the system to shift to reestablish a new equilibrium position.
How are the concentrations of reactants and products affected when a system is at equilibrium?
At equilibrium, the concentrations of reactants and products remain constant as the rate of the forward reaction is equal to the rate of the reverse reaction. This means that the amounts of reactants being converted to products is the same as the amounts of products being converted back to reactants, resulting in a dynamic balance where the concentrations of reactants and products do not change over time.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments