Chemistry Significant Figures Worksheet
Do you struggle with understanding significant figures in chemistry? If so, this Chemistry Significant Figures Worksheet can provide the perfect solution. By focusing on this specific topic, this worksheet aims to help students grasp the concept of significant figures and their importance in scientific measurements. Whether you're a high school student studying chemistry or a college student seeking to refresh your knowledge, this worksheet is designed to help you gain confidence in working with significant figures.
Table of Images 👆
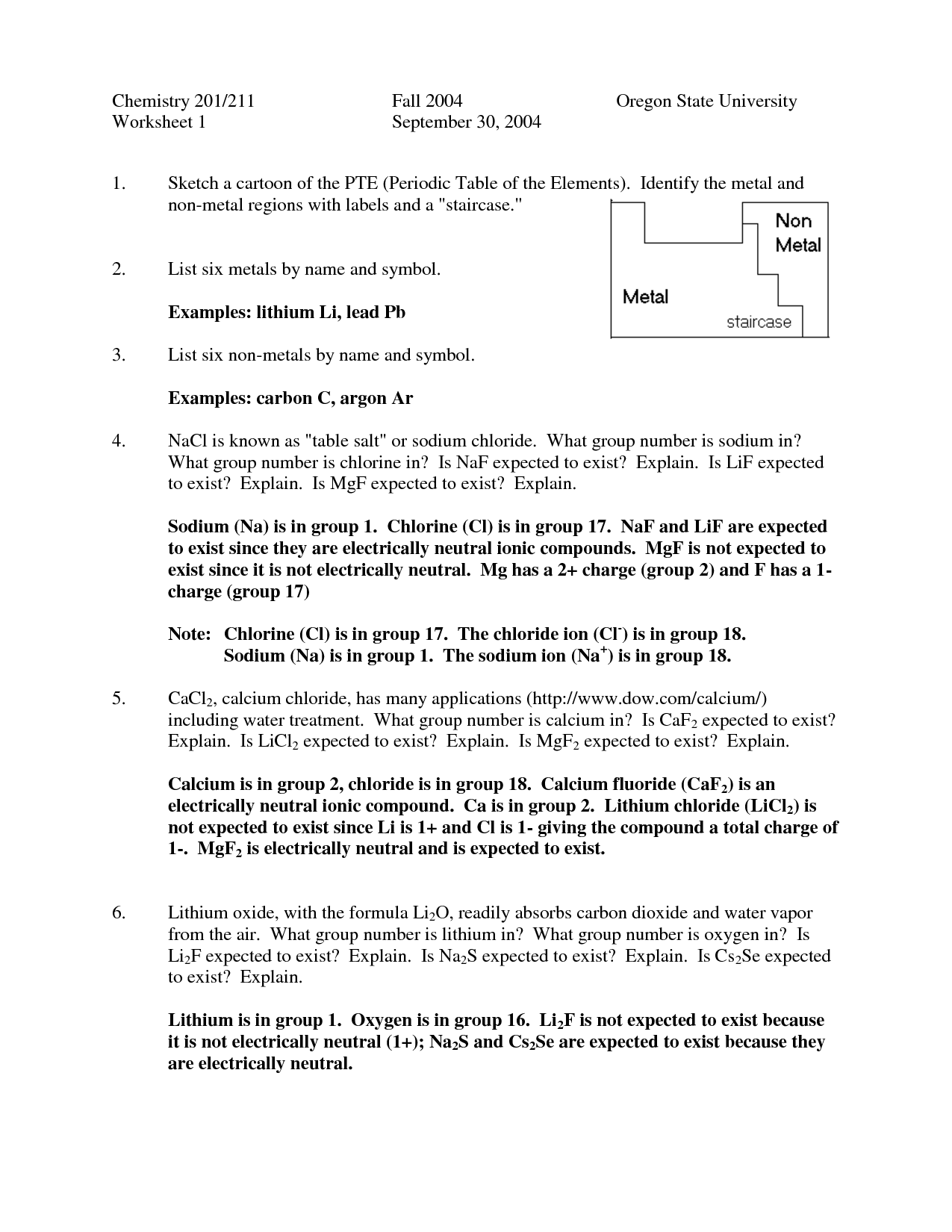
- Significant Figures Worksheet and Answer Key
- Significant Figures Worksheet Answers
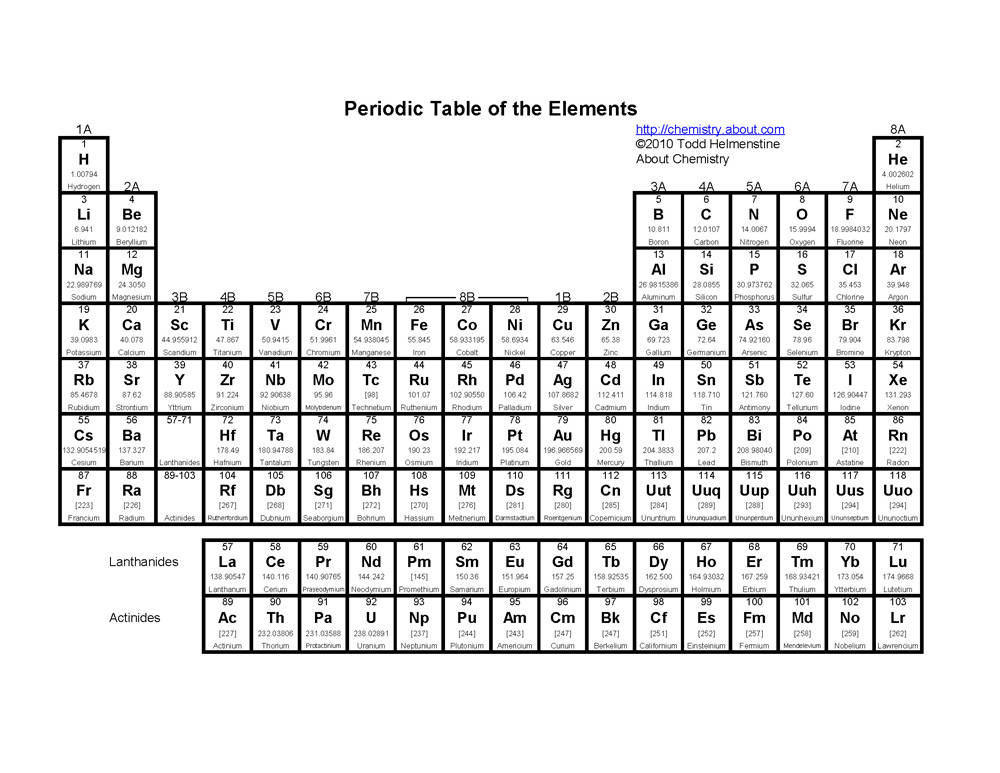
- Periodic Table Coloring Worksheet
- Physical vs Chemical Change Worksheet
- Chemistry Worksheet Matter 1 Answer Key
- Periodic Table Worksheet Answers
- Types of Chemical Reactions Worksheet Answers
- Element Worksheet Answer Key
- Electromagnetic Spectrum Worksheet
- Nomenclature Worksheet Answers
- The New Testament
More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What are significant figures in chemistry?
Significant figures in chemistry refer to the digits in a numerical value that carry meaning and provide precision. They include all certain digits plus one estimated digit. Significant figures are important in measurements and calculations to indicate the level of uncertainty or precision in a given value, and they help ensure the accuracy of scientific data and results.
How do you determine the number of significant figures in a measurement?
To determine the number of significant figures in a measurement, you count all the digits that are known with certainty, plus one estimated digit. Non-zero digits, zeros between non-zero digits, and zeros at the end of a number after a decimal point are considered significant. Trailing zeros in a whole number without a decimal point may or may not be significant, depending on how the measurement is made. Scientific notation is another method to indicate the number of significant figures explicitly.
What is the significance of significant figures in scientific calculations?
Significant figures are crucial in scientific calculations because they indicate the precision of measured values. By using the appropriate number of significant figures, scientists can communicate the accuracy of a measurement and ensure that their calculations reflect the limits of experimental uncertainty. This helps to prevent misinterpretation of data and promotes transparency in scientific research and analysis.
How do you round off numbers to the appropriate number of significant figures?
To round off numbers to the appropriate number of significant figures, count the number of significant figures in the original number. Then, identify the last significant figure you want to keep and look at the next digit. If it's 5 or greater, round the last significant figure up by one. If it's less than 5, leave the last significant figure unchanged. Finally, replace all the digits to the right of the last significant figure with zeros.
What are the rules for determining significant figures in addition and subtraction calculations?
When adding or subtracting numbers, the result should be rounded to the same decimal place as the number with the least number of decimal places in the calculation. For example, if you are adding 5.23 + 7.961, you would round your answer to the tenths place as 5.2 + 7.9 = 13.1. The number of significant figures in the final answer should then match the number of significant figures in the number with the least number of decimal places, which in this case is 2.
What are the rules for determining significant figures in multiplication and division calculations?
When multiplying or dividing numbers, the result should have the same number of significant figures as the factor or dividend with the fewest significant figures. For multiplication, count the significant figures in each number and pick the smallest count. For division, count the significant figures in the dividend and divisor and again pick the smallest count. The final answer should be rounded to that smallest count to maintain the appropriate level of precision.
How do you apply significant figures when performing mathematical operations with measured values?
When performing mathematical operations with measured values, you should apply significant figures by following these rules: 1) Perform the calculation using all the digits provided in the measurements; 2) Round the final answer to the least number of significant figures present in the original measurements; 3) For addition and subtraction, round the answer to the least number of decimal places present in the original measurements; 4) For multiplication and division, round the answer to the least number of significant figures in the original measurements. By following these guidelines, you can maintain accuracy and precision in your calculations while properly handling significant figures.
Why is it important to report measurements with the correct number of significant figures?
Reporting measurements with the correct number of significant figures is important because it ensures precision and accuracy in scientific and mathematical calculations. By using the appropriate number of significant figures, we convey the level of certainty in our measurements and calculations. Incorrectly reporting significant figures can lead to errors in data analysis and misinterpretation of results. It is essential to maintain consistency and adhere to the rules of significant figures to uphold the integrity and reliability of scientific findings.
How do you handle zeros in significant figures?
Zeros in significant figures are counted as significant if they are between non-zero digits or at the end of a number with a decimal point. Leading zeros are not counted as significant figures. When performing calculations with significant figures, it is important to pay attention to zeros to ensure accuracy and precision in the final result.
What are the potential sources of error when dealing with significant figures in chemistry measurements?
The potential sources of error when dealing with significant figures in chemistry measurements include limitations of the measuring instrument used, uncertainties in reading the measurement scale, human error in recording or interpreting data, improper calibration of equipment, rounding errors in calculations, and inconsistencies in the number of significant figures provided in the data used for calculations. It is essential to consider and minimize these sources of error to ensure accurate and reliable measurement data with the appropriate number of significant figures.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.























Comments