Chemistry Conversion Factors Worksheet
Are you a high school or college student looking for a helpful tool to aid in your understanding of chemistry conversion factors? Look no further! This chemistry conversion factors worksheet is designed to assist you in practicing and mastering the essential concepts of unit conversions in chemistry. With a variety of problems involving different units and measurements, this worksheet will help you become more comfortable and confident with converting between different quantities in the fascinating world of chemistry.
Table of Images 👆
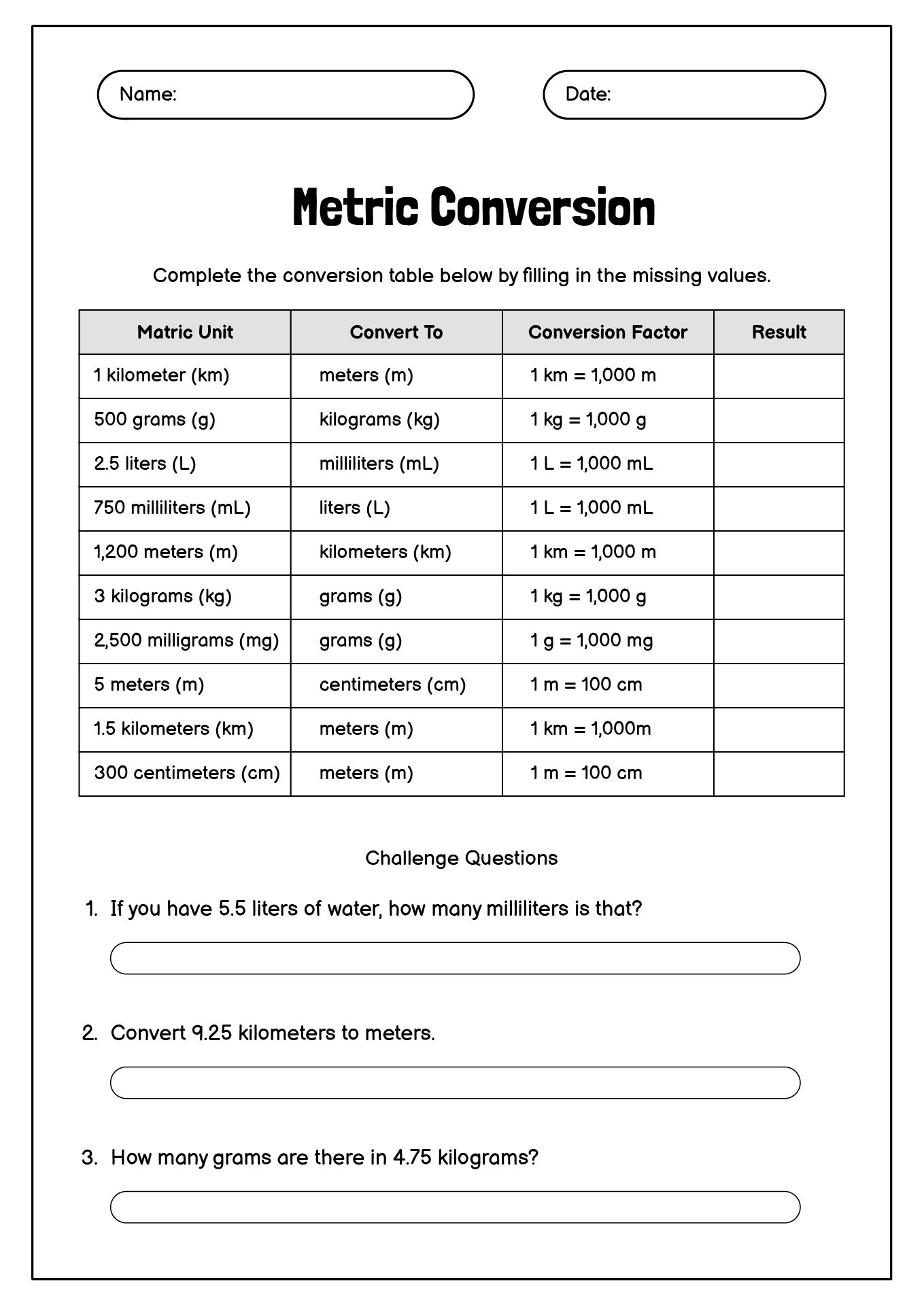
- Metric Conversion Table Chart
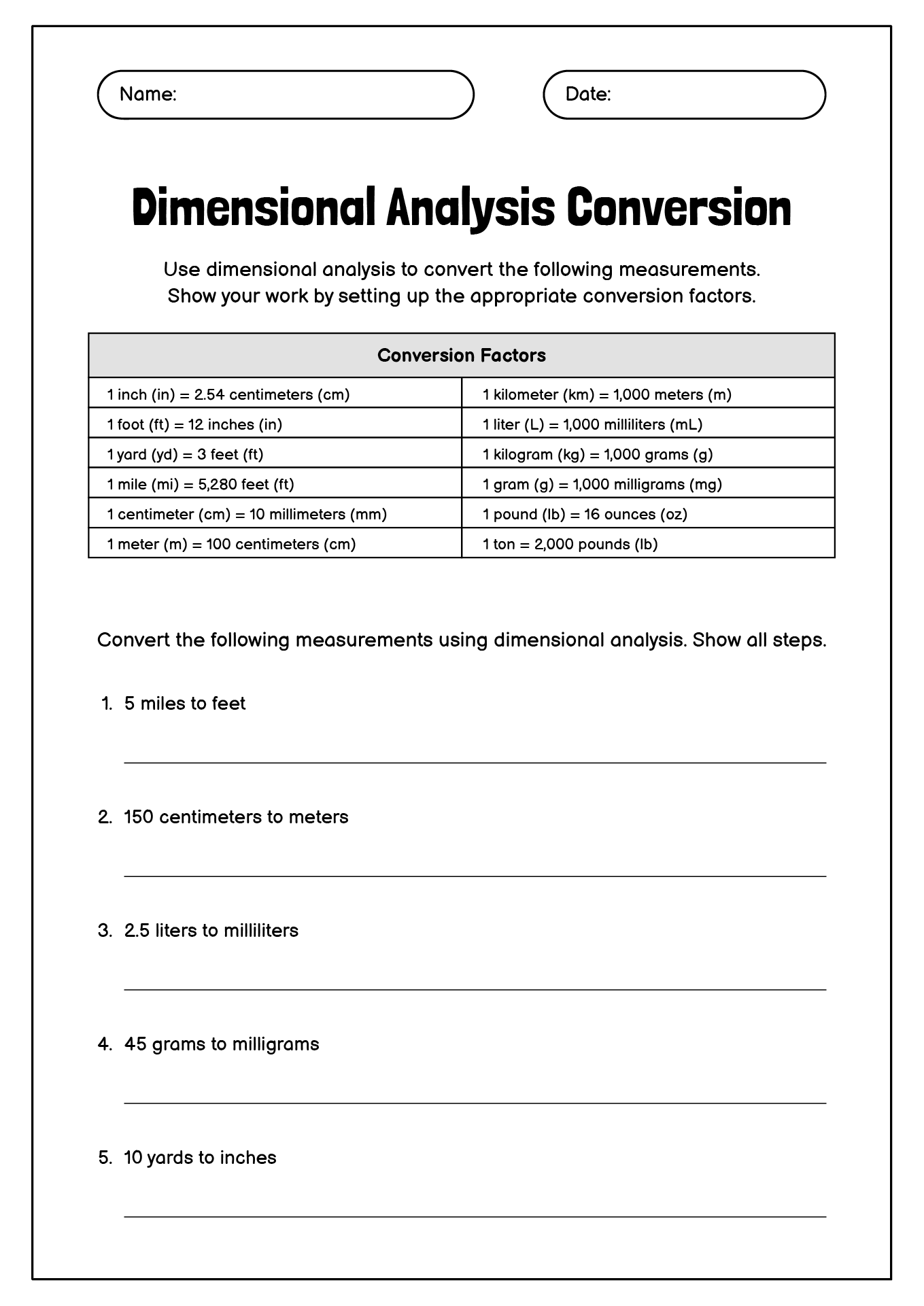
- Dimensional Analysis Conversion Chart
- Chemistry Conversion Factors Lesson Worksheet
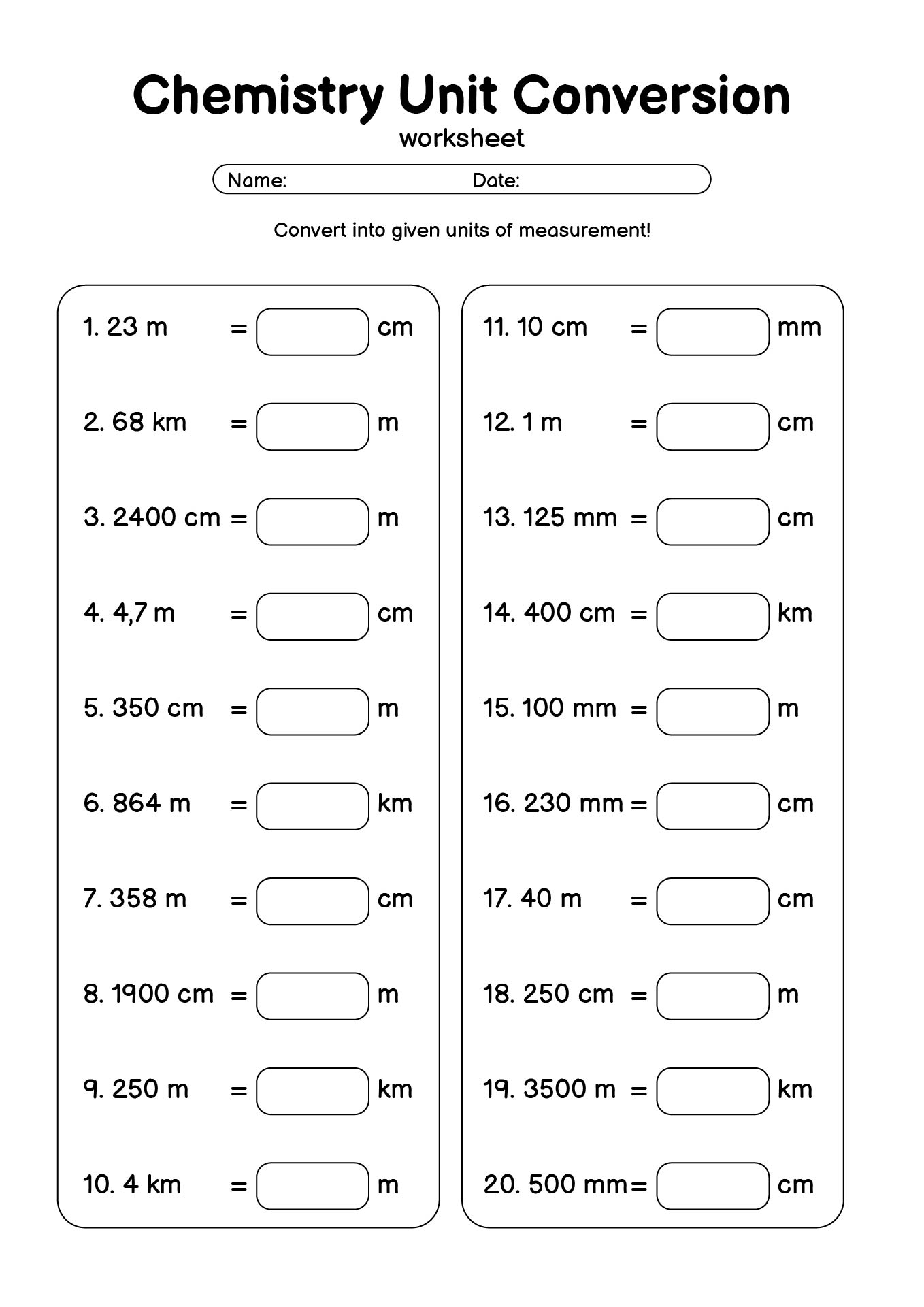
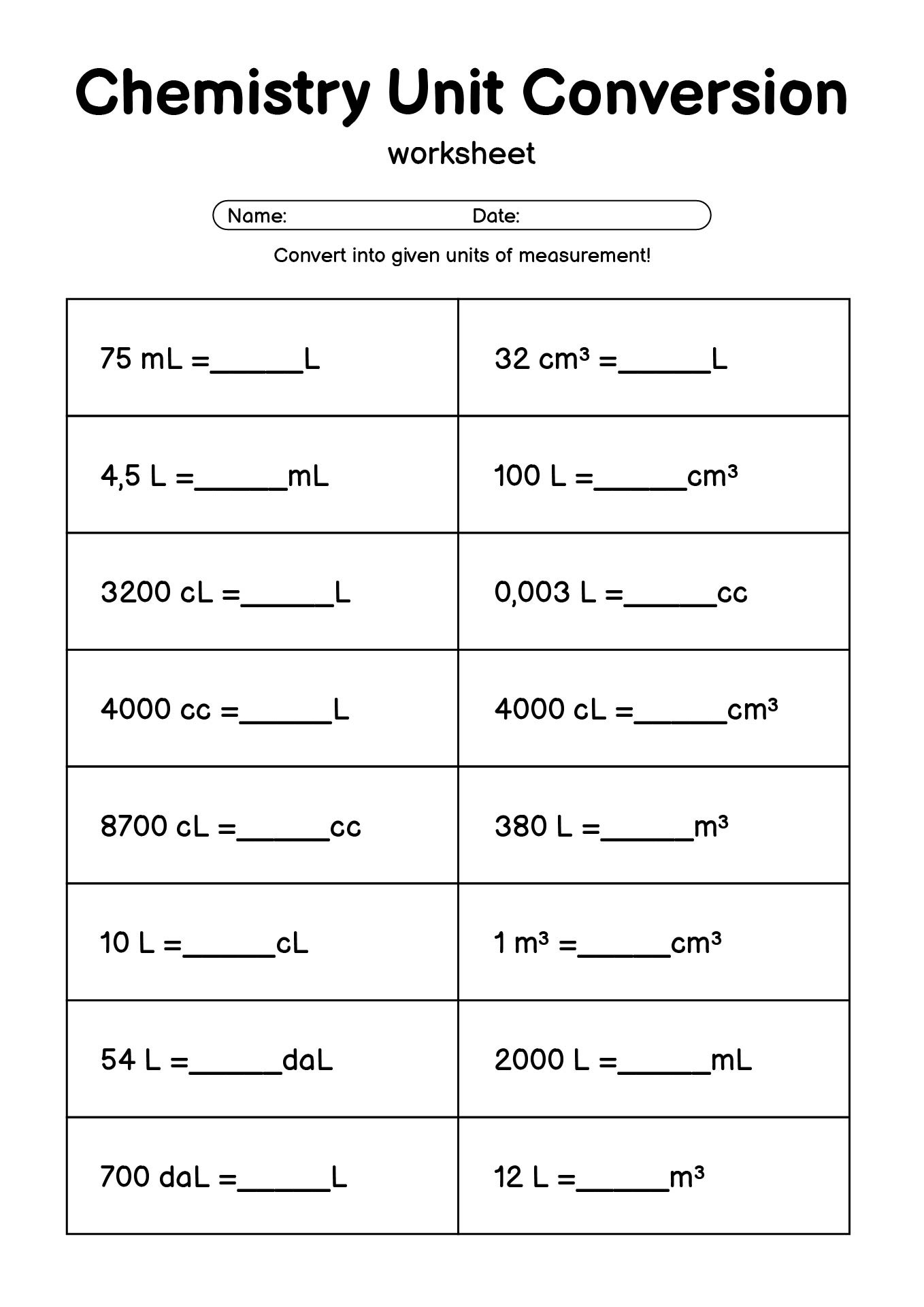
- Chemistry Unit Conversion Practice Worksheet
- Chemistry Conversion Factors Practice Worksheet
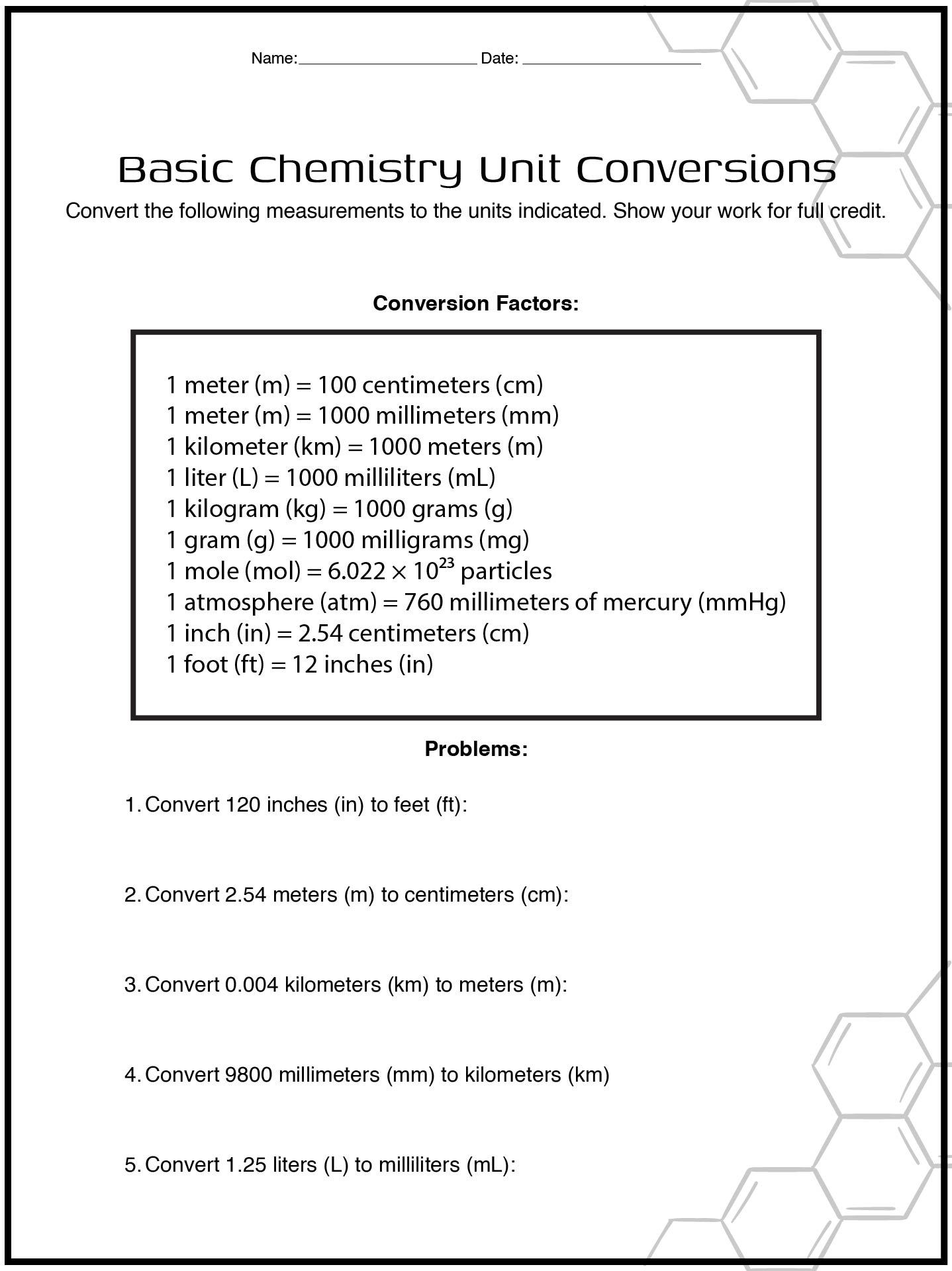
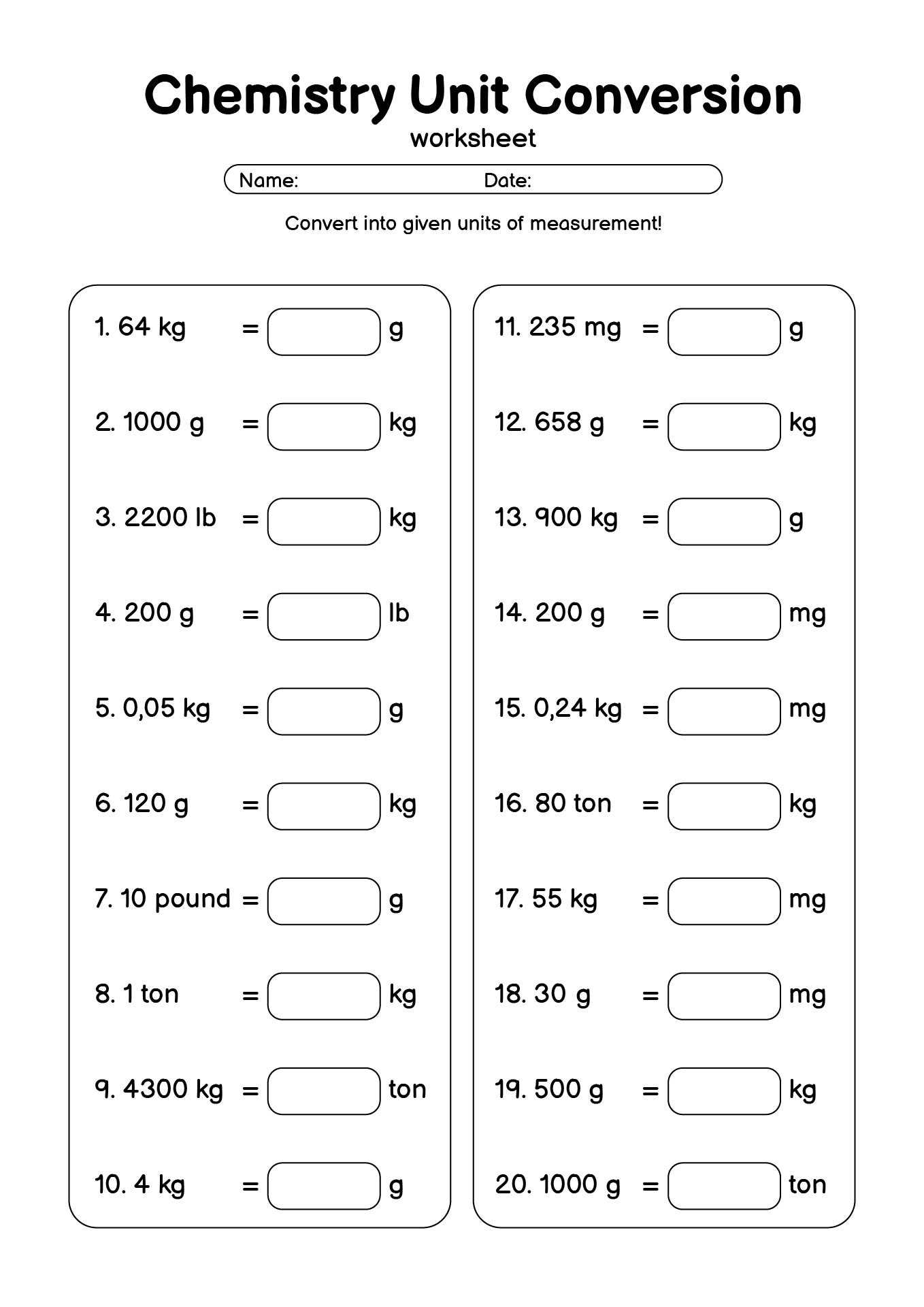
- Basic Chemistry Unit Conversion Worksheet
- Chemistry Unit Conversion Practice Worksheet
- Basic Chemistry Conversion Factors Sheet
- High School Chemistry Conversion Exercises
- Introductory Chemistry Conversion Worksheet
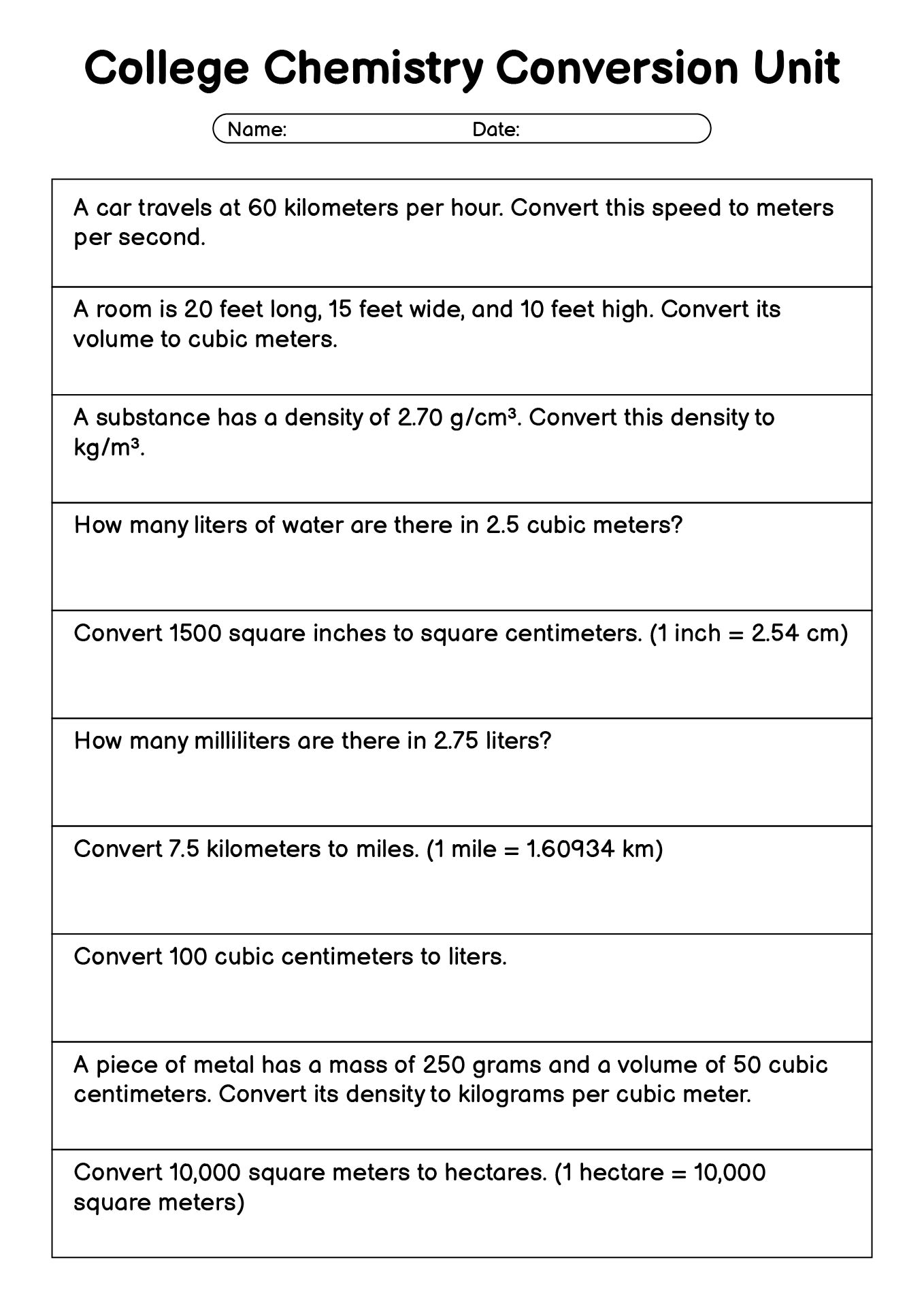
- College Chemistry Unit Conversion Problems
- General Chemistry Conversion Factors Challenge
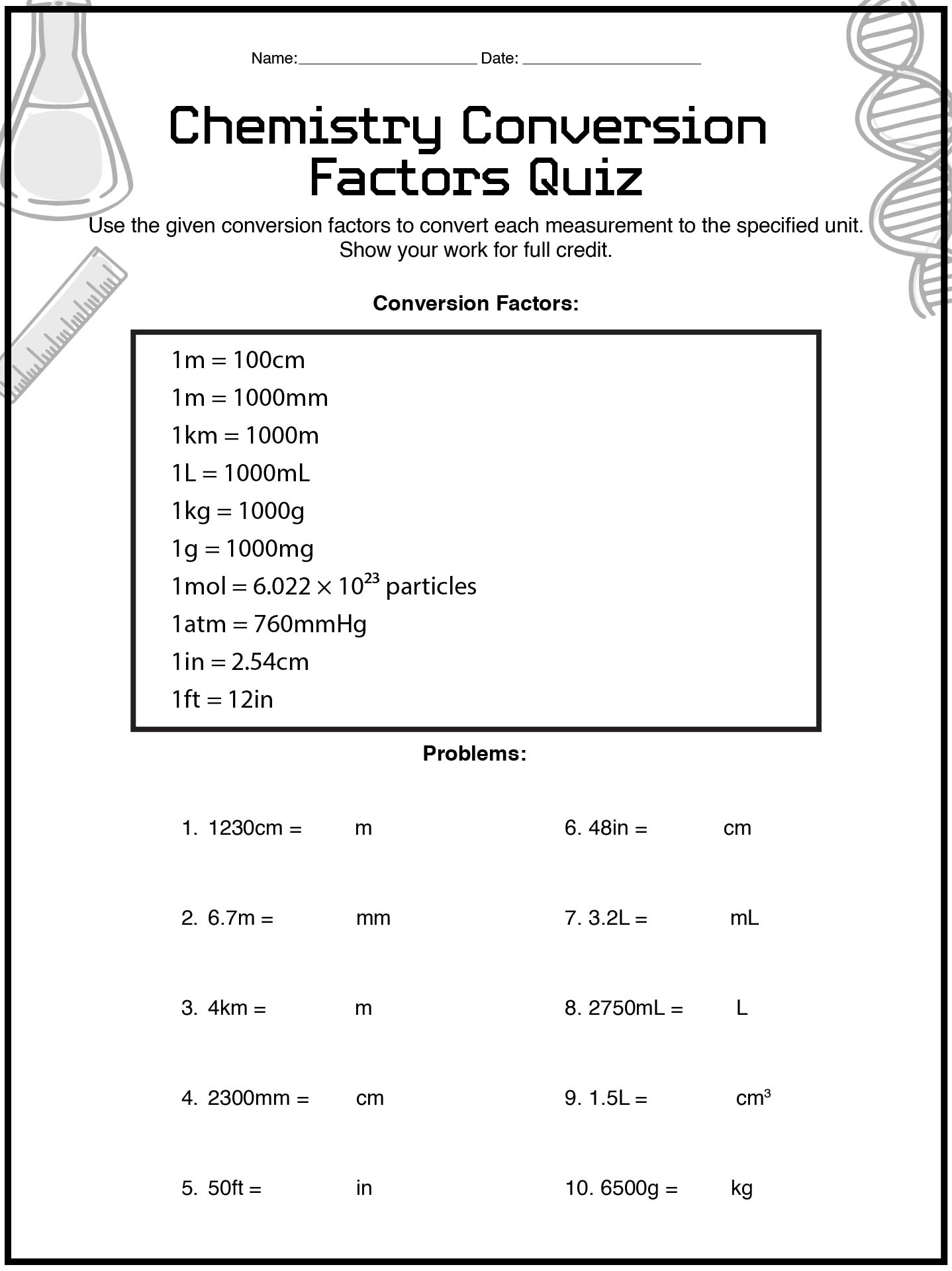
- Chemistry Conversion Factors Quiz Worksheet
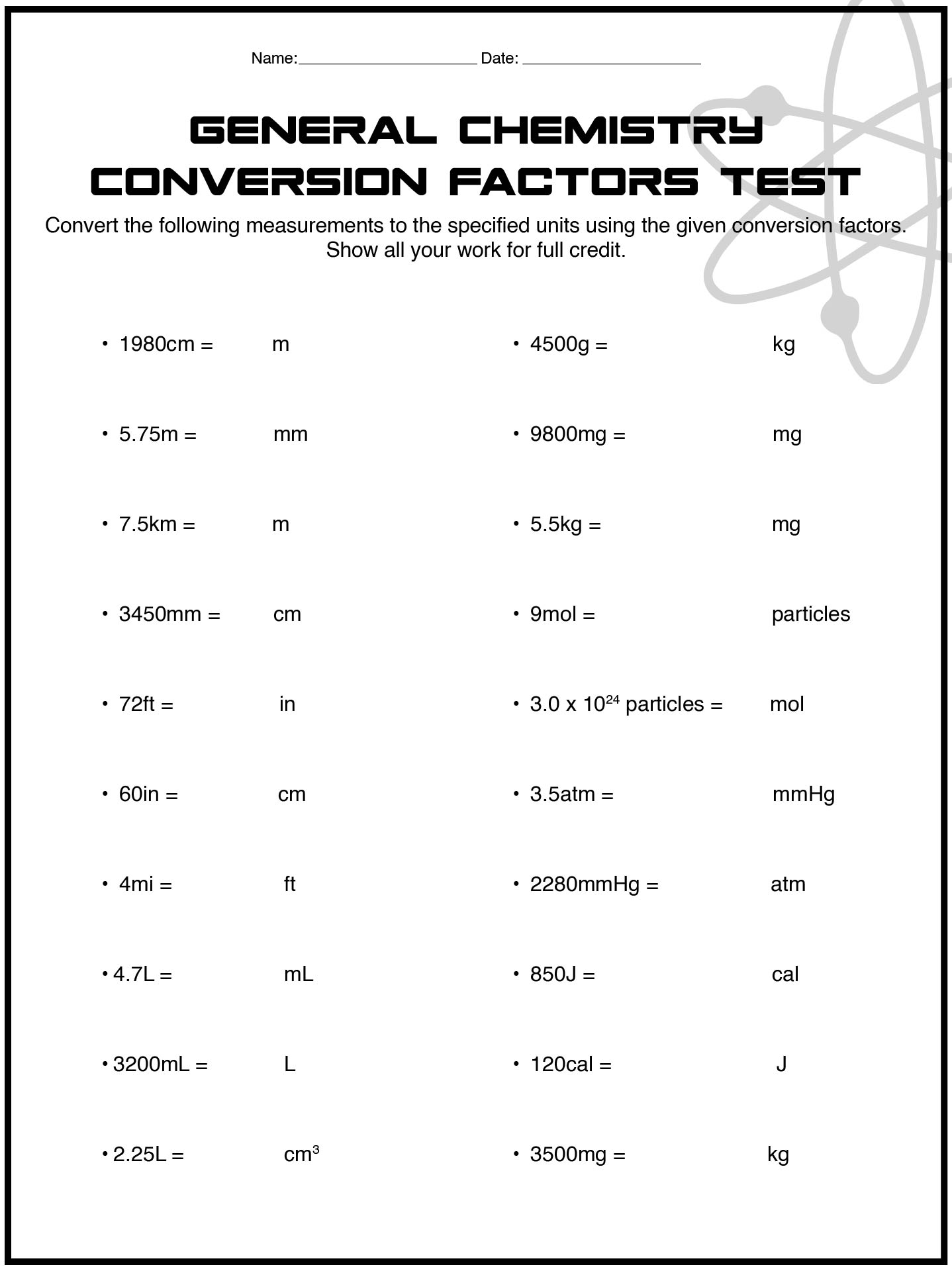
- General Chemistry Conversion Factors Test Worksheet

More Chemistry Worksheets
Chemistry Lab Equipment WorksheetChemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
How many grams are in 1 mole of a substance?
The number of grams in 1 mole of a substance is equal to its molar mass, which is the atomic mass of the element or the sum of the atomic masses for a compound. For example, the molar mass of carbon (C) is approximately 12 grams per mole, while the molar mass of water (H2O) is approximately 18 grams per mole.
How many liters are in 1 mole of a gas at standard temperature and pressure (STP)?
At standard temperature and pressure (STP), 1 mole of any ideal gas occupies approximately 22.4 liters.
How many atoms are in 1 mole of an element?
Avogadro's number represents the number of atoms in 1 mole of an element, which is approximately 6.022 x 10^23 atoms.
How many moles are in 1 gram of a substance?
The number of moles in 1 gram of a substance depends on the substance's molar mass. To find the number of moles, you divide the given mass (1 gram) by the molar mass of the substance. This calculation allows you to determine the amount of substance present in terms of moles.
How many moles of oxygen are required to completely react with 2 moles of hydrogen gas to form water?
One mole of oxygen is required to react with 2 moles of hydrogen gas to form water. This is based on the chemical equation for the reaction of hydrogen and oxygen to form water, which is 2H2 + O2 ? 2H2O.
How many milliliters are in 1 liter?
There are 1000 milliliters in 1 liter.
How many grams of carbon dioxide are produced when 5 moles of methane (CH4) react with excess oxygen?
When 5 moles of methane react with excess oxygen to produce carbon dioxide, the balanced chemical equation shows that 1 mole of methane produces 1 mole of carbon dioxide. Therefore, 5 moles of methane will produce 5 moles of carbon dioxide. The molar mass of carbon dioxide is 44.01 g/mol, so 5 moles of carbon dioxide would be 5 x 44.01 = 220.05 grams of carbon dioxide produced.
How many grams of sodium chloride (NaCl) are in 250 mL of a 0.2 M NaCl solution?
To calculate the number of grams of NaCl in a 0.2 M solution, we need to use the molarity formula: moles = Molarity x Volume (L). Since the given volume is 250 mL (which is 0.25 L) and the molarity is 0.2 M, we can calculate moles of NaCl as 0.2 x 0.25 = 0.05 moles. Next, we convert moles to grams by multiplying by the molar mass of NaCl (58.44 g/mol): 0.05 moles x 58.44 g/mol = 2.92 grams of NaCl in 250 mL of 0.2 M NaCl solution.
How many grams are in 1 amu (atomic mass unit)?
One atomic mass unit (amu) is equivalent to 1.66053906660 x 10^-24 grams.
How many molecules are in 0.5 moles of water (H2O)?
There are 3.011 x 10^23 molecules in 0.5 moles of water (H2O).
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



















Comments