Balancing Equations Worksheet Answer Key
Are you a student or teacher looking for a helpful resource to practice balancing equations? Look no further! In this blog post, we will provide you with a comprehensive balancing equations worksheet answer key. This worksheet is designed for individuals who are learning or reviewing the concept of balancing chemical equations.
Table of Images 👆
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Answer Key
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Answers
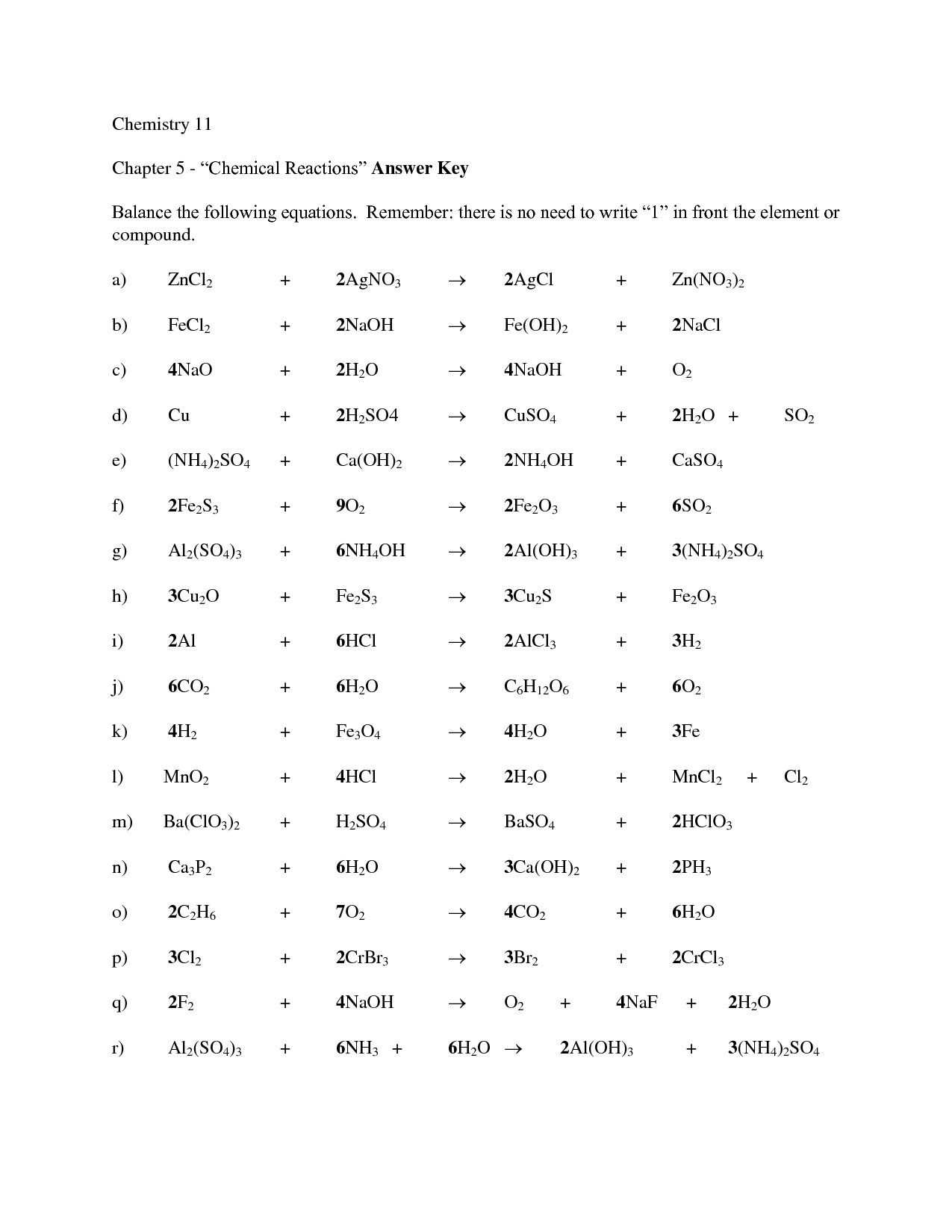
- Balancing Chemical Equations Worksheet Answer Key
- Balancing Chemical Equations Worksheet 1 Answers
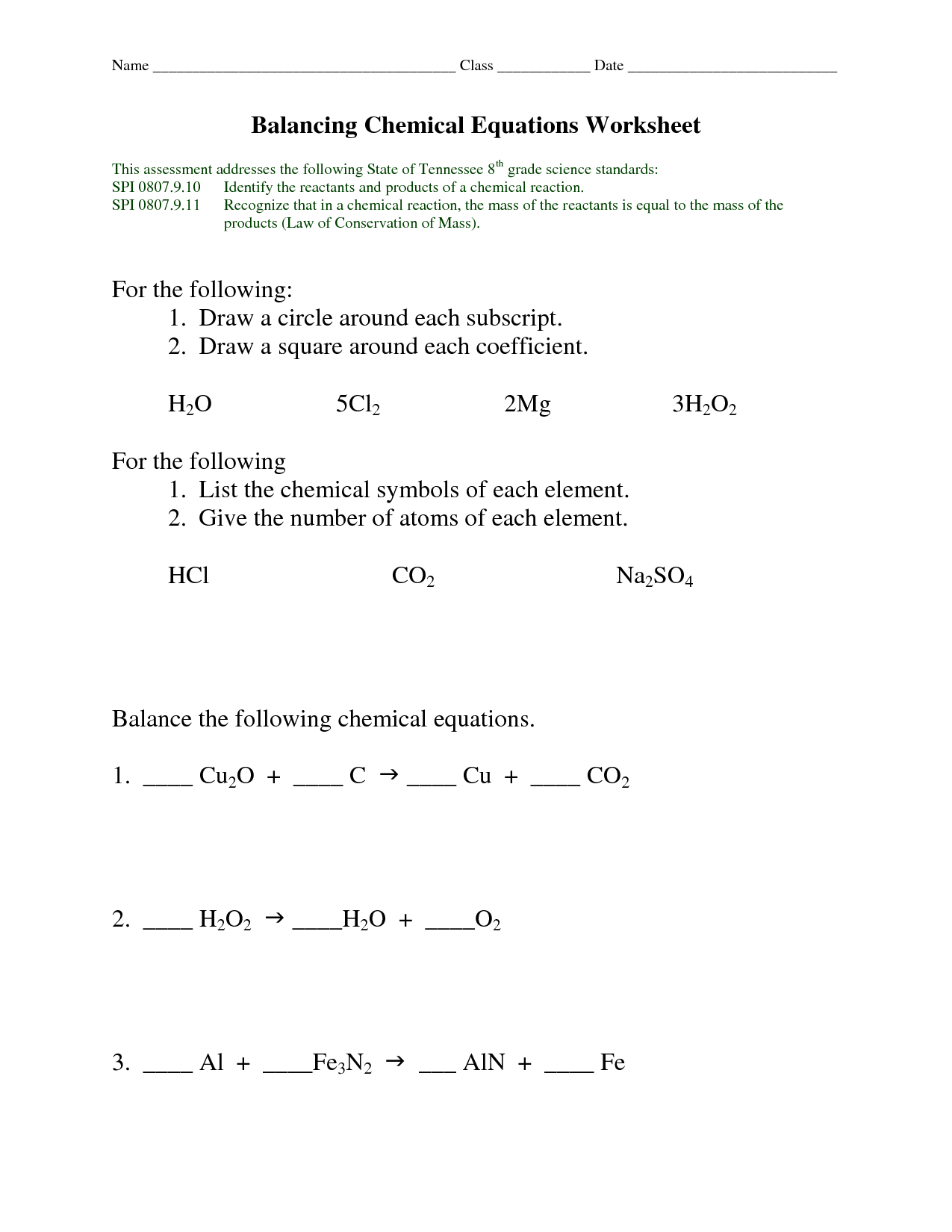
- Balancing Chemical Equations Worksheet
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Key
- Balancing Chemical Equations Worksheet Answer Key
- Balancing Chemical Equations Worksheet Answers
- Balancing Equations Worksheet Answers
- Writing Chemical Equations Worksheet Answers Balancing
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is a balancing equation?
A balancing equation is the process of adjusting the coefficients of reactants and products in a chemical equation to ensure that the number of each type of atom is the same on both sides of the equation, adhering to the law of conservation of mass. This is crucial for accurately representing chemical reactions and understanding the stoichiometry of the reaction.
What is the purpose of balancing equations?
The purpose of balancing equations in chemistry is to ensure that the number of atoms of each element present in the reactants is equal to the number of atoms of that element in the products. Balancing equations helps maintain the law of conservation of mass, which dictates that matter cannot be created or destroyed in a chemical reaction but only rearranged. By balancing equations, scientists can accurately represent the chemical reactions that occur and predict the amounts of reactants and products involved in a reaction.
How do you determine the coefficients of an equation?
To determine the coefficients of an equation, look at the numerical values that precede the variables in each term. The coefficient is the number that multiples with the variable. For example, in the equation 3x + 2y - 5z = 10, the coefficients are 3, 2, and -5 for x, y, and z respectively. Identify these numbers to find the coefficients of the equation.
Why is it important to balance equations in chemistry?
Balancing equations in chemistry is important because it ensures that the law of conservation of mass is followed in chemical reactions. By balancing equations, we are able to accurately represent the ratio of reactants and products involved in a reaction, which helps in predicting the amount of substances produced or consumed. This is crucial for performing accurate calculations and understanding the chemical behavior of substances.
What are some common methods used to balance equations?
Some common methods used to balance chemical equations include inspection method, oxidation-number method, half-reaction method, and algebraic method. Each method involves manipulating coefficient values of reactants and products in order to ensure the conservation of mass and atoms on both sides of the chemical equation.
Can you provide an example of a balanced chemical equation?
Sure! An example of a balanced chemical equation is the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). The balanced equation for this reaction is 2H2 + O2 -> 2H2O. This equation shows that two molecules of hydrogen combine with one molecule of oxygen to produce two molecules of water, where the number of atoms of each element on both sides of the reaction arrow is equal, demonstrating the principle of conservation of mass.
What are the guidelines for balancing equations?
When balancing chemical equations, the goal is to ensure that the number of atoms of each element is the same on both sides of the equation. Start by identifying the elements present in the reactants and products, then adjust the coefficients of the compounds to balance the number of atoms. Follow these steps: 1. Write down the unbalanced equation. 2. Balance the atoms of elements that appear in only one reactant and one product first. 3. Balance other elements that appear in multiple compounds on each side. 4. Adjust coefficients as needed, but never change subscripts in a formula. 5. Double-check that all atoms are balanced after making adjustments.
What happens if an equation is not properly balanced?
If an equation is not properly balanced, it means that the number of atoms of each element is not equal on both sides of the equation. This can lead to incorrect results when solving the equation, as it violates the law of conservation of mass, which states that mass cannot be created or destroyed in a chemical reaction. Properly balancing an equation is crucial to accurately represent the reactions that take place in a chemical reaction.
How can you tell if an equation is balanced?
An equation is balanced if the number of atoms of each element is the same on both sides of the equation. This is achieved by adjusting the coefficients in front of each compound so that the total mass of the reactants equals the total mass of the products. Balancing equations is typically done by trial and error, manipulating the coefficients until the equation is balanced.
Can you explain the concept of stoichiometry in relation to balancing equations?
Stoichiometry is the calculation of the quantities of reactants and products in a chemical reaction based on the balanced equation. When balancing equations, coefficients are used to represent the relative amounts of each substance involved in the reaction. This is crucial for determining the exact amounts of reactants needed and products produced in a chemical reaction. Stoichiometry helps ensure that mass is conserved and that the reaction follows the correct proportions of substances as defined by the balanced equation.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.





























Comments