Balancing Chemical Equations Worksheet 1
Balancing chemical equations can often be a challenging and daunting task for students studying chemistry. With the purpose of providing a helpful and effective learning tool, the Balancing Chemical Equations Worksheet 1 offers a carefully designed set of problems for students to practice and enhance their understanding of this important concept. This worksheet serves as a valuable resource for students seeking to improve their skills in balancing chemical equations and reinforce their knowledge of the subject matter.
Table of Images 👆
- Balancing Chemical Equations Worksheet Answers
- Balancing Chemical Equations Worksheet Answers
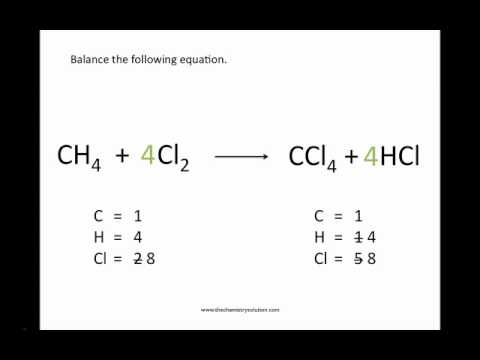
- Practice Balancing Chemical Equations Worksheet
- Balancing Chemical Equations Worksheet Answers
- Types Chemical Reactions Worksheets Answers
- Balancing Chemical Equations Answer Key
- Balancing Chemical Equations Worksheet
- Balancing Equations Worksheet Answers
- Balancing Chemical Equations Examples
- Lewis Dot Structure Worksheet Answers
- Classifying Chemical Reactions Worksheet Answer Key
- Percent Yield Calculations Worksheet
- Balancing Chemical Equations Worksheet Answers
- Student Exploration Chemical Equations Gizmo Answer Key
- Double Replacement Reaction Worksheet
- Smores Stoichiometry Lab
- Smores Stoichiometry Lab
- Smores Stoichiometry Lab
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is a chemical equation?
A chemical equation is a symbolic representation of a chemical reaction using chemical formulas and symbols to indicate the reactants and products involved in the reaction as well as the physical states and conditions of the reaction.
How do you determine the coefficient of a reactant or product in a chemical equation?
To determine the coefficient of a reactant or product in a chemical equation, you balance the equation by ensuring that the number of atoms for each element is equal on both sides of the reaction. Start by writing down the unbalanced equation and adjusting the coefficients of the compounds until the number of each type of atom is the same on both sides. This process involves trial and error while following the law of conservation of mass.
What is the law of conservation of mass?
The law of conservation of mass states that in a closed system, mass cannot be created or destroyed, it can only change form or be transferred from one place to another. This means that the total mass of a system remains constant regardless of any physical or chemical changes that may occur within that system.
Why is it important to balance chemical equations?
Balancing chemical equations is important because it ensures that the law of conservation of mass is obeyed, meaning that the same number and types of atoms are present on both sides of the equation. This is crucial for accurately predicting the outcome of chemical reactions and understanding the stoichiometry, which is the quantitative relationship between reactants and products in a chemical reaction. By balancing chemical equations, scientists can determine the precise amounts of reactants needed and products produced, helping them to conduct experiments more effectively and to make reliable calculations in various fields of chemistry.
How do you balance chemical equations using the trial and error method?
To balance chemical equations using the trial and error method, start by balancing the most complex or uncommon element first. Then adjust the coefficients of each compound in the equation, keeping track of the total number of atoms on each side. Gradually work through the equation, changing coefficients until the number of each type of atom is equal on both sides. This may involve multiple attempts and adjustments until a balanced equation is achieved.
What is the role of coefficients in balancing chemical equations?
Coefficients in chemical equations are used to balance the number of atoms of each element on both the reactant and product sides. By adjusting the coefficients, one can ensure that the law of conservation of mass is obeyed, meaning that the number of atoms of each element is the same on both sides of the equation. This ensures the equation accurately represents the reaction taking place.
Can you change the subscripts of elements to balance a chemical equation? Why or why not?
No, you cannot change the subscripts of elements to balance a chemical equation as this would alter the actual chemical formula of the compounds involved in the reaction. The coefficients in front of the chemical formulas are adjusted to balance the number of atoms of each element on both the reactant and product sides of the equation while keeping the chemical identities of the elements intact. Changing the subscripts would result in a different compound altogether with different chemical properties.
What are the common types of chemical reactions that need to be balanced?
Some common types of chemical reactions that need to be balanced include combustion reactions, synthesis reactions, decomposition reactions, single displacement reactions, and double displacement reactions. Balancing these reactions ensures that the law of conservation of mass is upheld, as each side of the chemical equation must contain the same number of atoms of each element.
Can you balance a chemical equation using fractions as coefficients? Why or why not?
No, fractions are not typically used as coefficients in balanced chemical equations because coefficients must represent whole number ratios of the number of moles of each reactant and product involved in the reaction. Using fractions would imply that a fraction of a molecule is participating in the reaction, which is not possible in a chemical reaction. Balanced chemical equations are derived based on the law of conservation of mass, where the number of atoms of each element must be the same on both sides of the equation.
How can you check if a chemical equation is balanced?
To check if a chemical equation is balanced, count the number of atoms of each element on both sides of the equation. Make sure that the number of each type of atom is the same on both sides by adjusting the coefficients in front of the chemical formulas. If the number of atoms is balanced for each element, the chemical equation is considered to be balanced.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



































Comments