Atomic Mass Worksheets with Answers
If you're a chemistry student or educator in search of reliable worksheets that cover the topic of atomic mass, you've come to the right place. In this blog post, we will explore a selection of atomic mass worksheets with detailed answer keys, specifically designed to reinforce your understanding of this fundamental concept. Whether you're a high school student preparing for an exam or a teacher looking for supplementary materials, these worksheets are here to assist you in your learning journey.
Table of Images 👆
- Atomic Number Worksheet
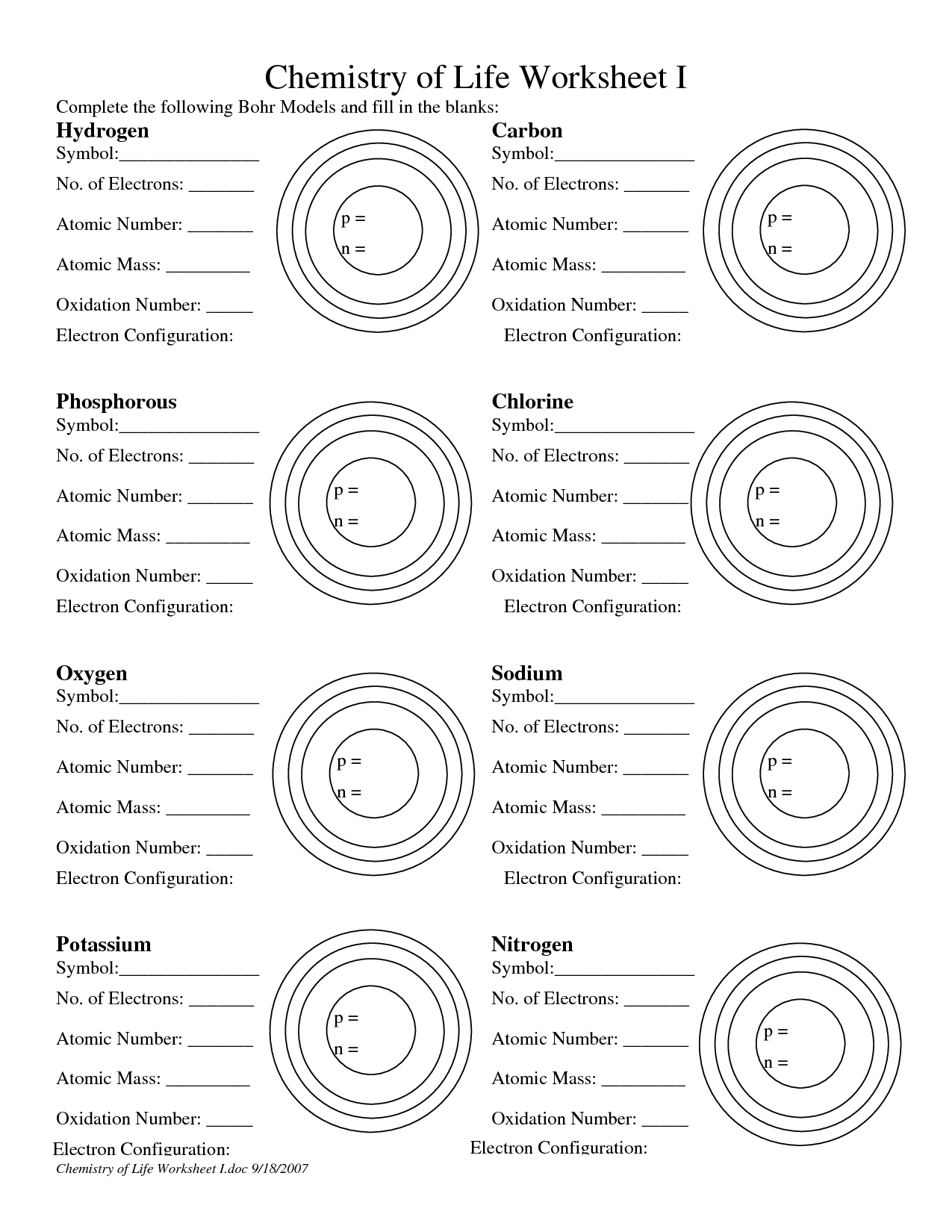
- Atomic Structure Bohr Model Worksheet
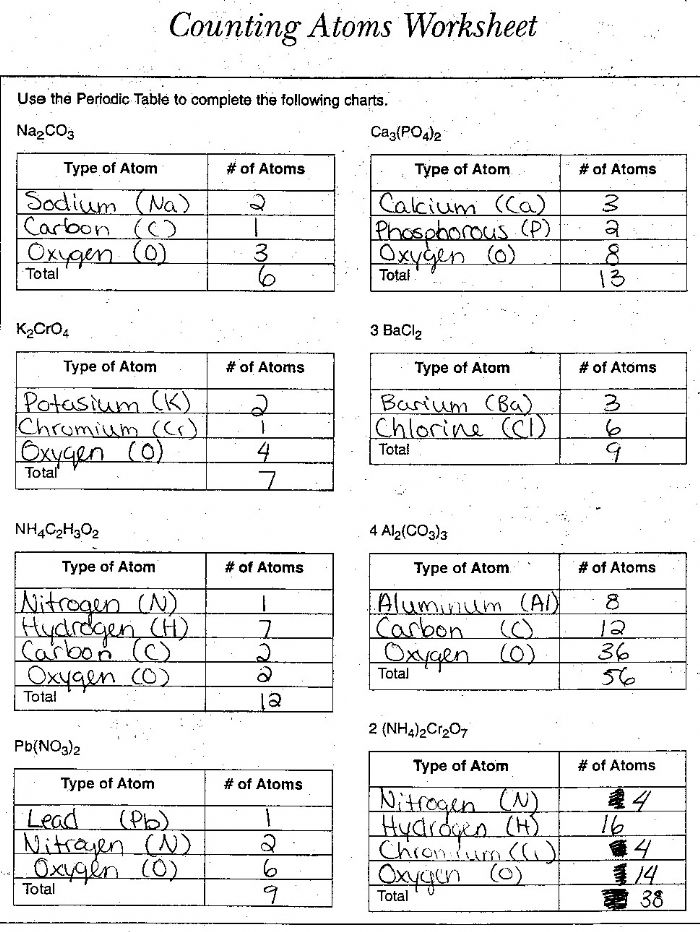
- Counting Atoms Worksheet Answer Key
- Lab Equipment Worksheet Answers
- Bonding Worksheet Answer Key
- Chemistry Worksheets with Answer Key
- Abundance of Isotopes Chem Worksheet 4 3 Answer Key
- Covering and Surrounding Math Book Answer Key
- Chemistry If8766 Worksheet Answer Key
- Chemistry Dimensional Analysis Worksheet
- Worksheets Answer Key
- Nuclear Reaction Equations Worksheet
- Naming Ionic Compounds Worksheet Answers
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Art Handouts and Worksheets
7 Elements of Art Worksheets
What is atomic mass?
Atomic mass is the total mass of an atom, which is the sum of the masses of protons, neutrons, and electrons present in the atom. It is typically expressed in unified atomic mass units (u) or daltons and is a key characteristic used to identify and compare different elements in the periodic table.
How is atomic mass calculated?
Atomic mass is calculated by taking the weighted average of the masses of all naturally occurring isotopes of an element, based on their relative abundance in nature. The atomic mass is often listed on the periodic table and is measured in atomic mass units (amu), where one amu is equal to one twelfth of the mass of a carbon-12 atom. This calculation takes into account the mass of each isotope and its abundance to give a more accurate representation of the average mass of an element.
Why is atomic mass expressed in atomic mass units (amu)?
Atomic mass is expressed in atomic mass units (amu) because it provides a convenient way to compare the masses of different atoms on a relative scale. By defining the mass of a single atom of carbon-12 as exactly 12 amu, scientists can easily determine the approximate mass of other atoms in relation to carbon-12. This standardized unit allows for consistency and simplicity in describing the masses of various elements and isotopes.
How does atomic mass differ from atomic number?
Atomic mass is the total mass of an atom, calculated by adding together the mass of protons, neutrons, and electrons, usually measured in atomic mass units. On the other hand, the atomic number is the number of protons in an atom, which uniquely defines an element and determines its chemical properties. So, while atomic mass considers the total mass of an atom, atomic number specifically refers to the number of protons in the nucleus of an atom.
What are isotopes and how do they affect atomic mass?
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons in their atomic nuclei. These different numbers of neutrons result in isotopes having different atomic masses. Since atomic mass is a weighted average of the masses of all the isotopes of an element, the presence of different isotopes will affect the overall atomic mass of the element. The atomic mass listed on the periodic table is a weighted average of the masses of all the naturally occurring isotopes of that element.
What is the relationship between atomic mass and the number of protons, neutrons, and electrons in an atom?
The atomic mass of an atom is determined by the sum of the number of protons and neutrons in its nucleus. The number of protons in an atom is equal to its atomic number, which determines the element the atom represents. Atoms are electrically neutral, so the number of electrons surrounding the nucleus is also equal to the number of protons. Therefore, the number of protons and neutrons in the nucleus, as well as the number of electrons in the electron cloud, collectively determine the atomic mass and identity of an atom.
Why is the concept of average atomic mass important in chemistry?
The concept of average atomic mass is important in chemistry because it helps scientists accurately calculate the mass of an element based on the abundance of its isotopes. This is crucial for understanding and predicting chemical reactions, as the atomic mass determines how elements interact with one another. Additionally, average atomic mass is used to identify elements and differentiate between isotopes, providing valuable information for various applications within the field of chemistry.
How do you determine the average atomic mass of an element with multiple isotopes?
To determine the average atomic mass of an element with multiple isotopes, you multiply the mass of each isotope by its natural abundance (in decimal form), then add up these values for each isotope. This calculation will give you the weighted average of all the isotopes' masses, representing the average atomic mass of the element.
How does the periodic table provide information about atomic mass?
The periodic table provides information about atomic mass by listing elements in order of increasing atomic number, which corresponds to the number of protons in an atom's nucleus. Elements are organized into rows and columns based on their properties, with elements in the same column having similar chemical characteristics. The atomic mass of each element is typically listed below its symbol on the periodic table, which is the average mass of an atom of that element compared to the mass of a carbon-12 atom. By looking at where an element is positioned on the periodic table, one can determine its atomic mass relative to other elements and make comparisons between different elements.
Why is accurate measurement of atomic mass crucial in scientific research and applications?
The accurate measurement of atomic mass is crucial in scientific research and applications because it allows scientists to determine the fundamental properties of elements, such as their reactivity, abundance, and isotopic composition. This information is essential for a wide range of scientific fields, including chemistry, physics, biology, and materials science. Additionally, accurate atomic mass measurements are important in fields such as nuclear energy, environmental monitoring, and pharmaceutical development, where precise knowledge of atomic properties is necessary for safety, efficiency, and efficacy.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments