Acid Nomenclature Worksheet
Are you struggling with understanding acid nomenclature? Look no further! This blog post is designed to help beginners grasp the basics of acid nomenclature and build their knowledge step by step. In this post, we will break down the different components of acid naming and provide examples to ensure a thorough understanding of the subject. So, if you are a chemistry student looking to improve your understanding of acid nomenclature, this post is for you!
Table of Images 👆
- Naming Acids Worksheet Answer Key
- Naming Binary Covalent Compounds Worksheet Answers
- Acids and Bases Worksheet Answers
- Naming Covalent Compounds Worksheet
- Chemical Nomenclature Worksheet Answers
- Naming Acids and Bases Worksheet Answers
- Naming Acids and Bases Worksheet Answer Key
- Chemical Nomenclature Worksheet
- Naming Binary Ionic Compounds Worksheet Answers
- Naming Acids and Bases Worksheet Answers
- Chemistry Naming Acids Worksheet
- Naming Acids and Bases Worksheet Answers
- Naming Ionic and Covalent Compounds Worksheet
More Other Worksheets
Kindergarten Worksheet My RoomSpanish Verb Worksheets
Healthy Eating Plate Printable Worksheet
Cooking Vocabulary Worksheet
My Shadow Worksheet
Large Printable Blank Pyramid Worksheet
Relationship Circles Worksheet
DNA Code Worksheet
Meiosis Worksheet Answer Key
Rosa Parks Worksheet Grade 1
What is acid nomenclature?
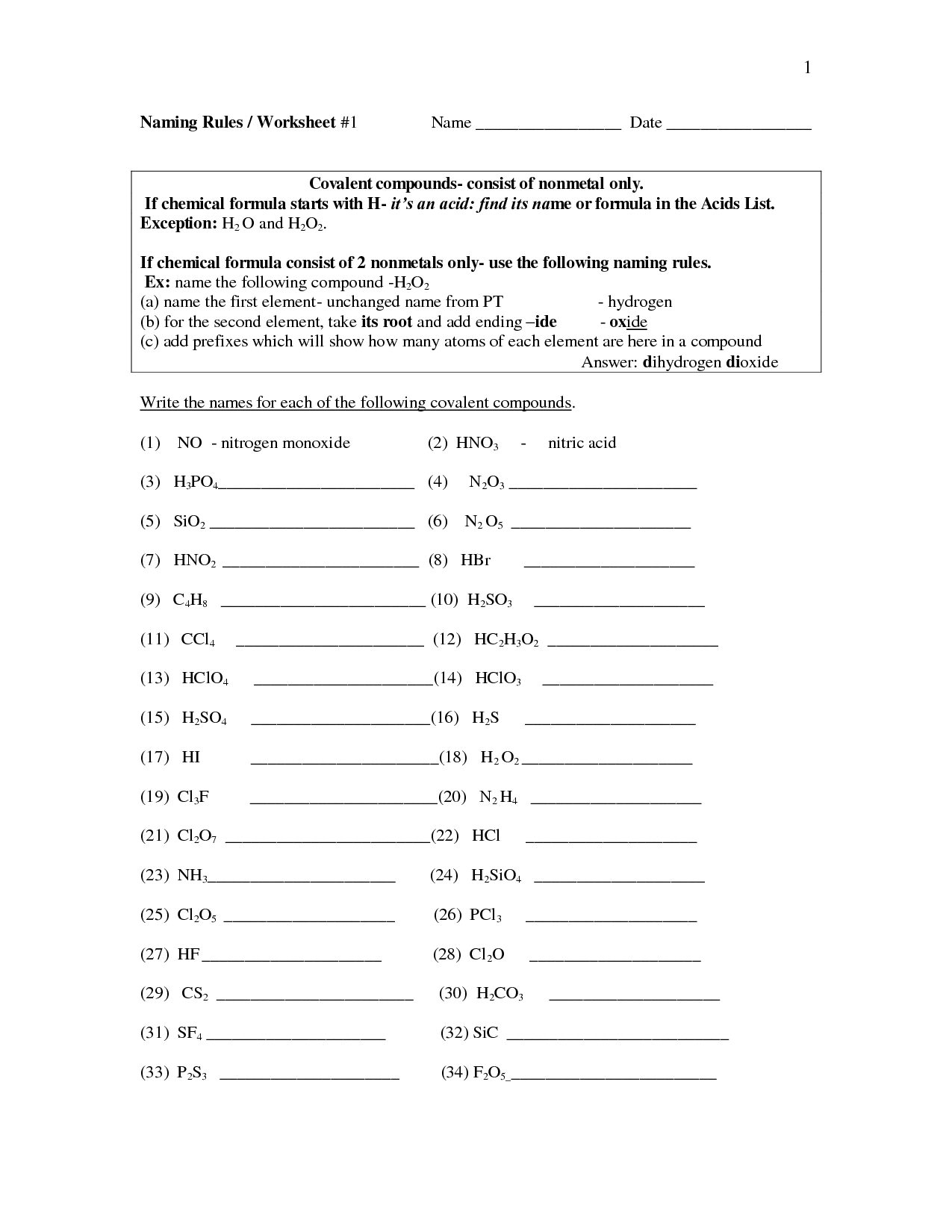
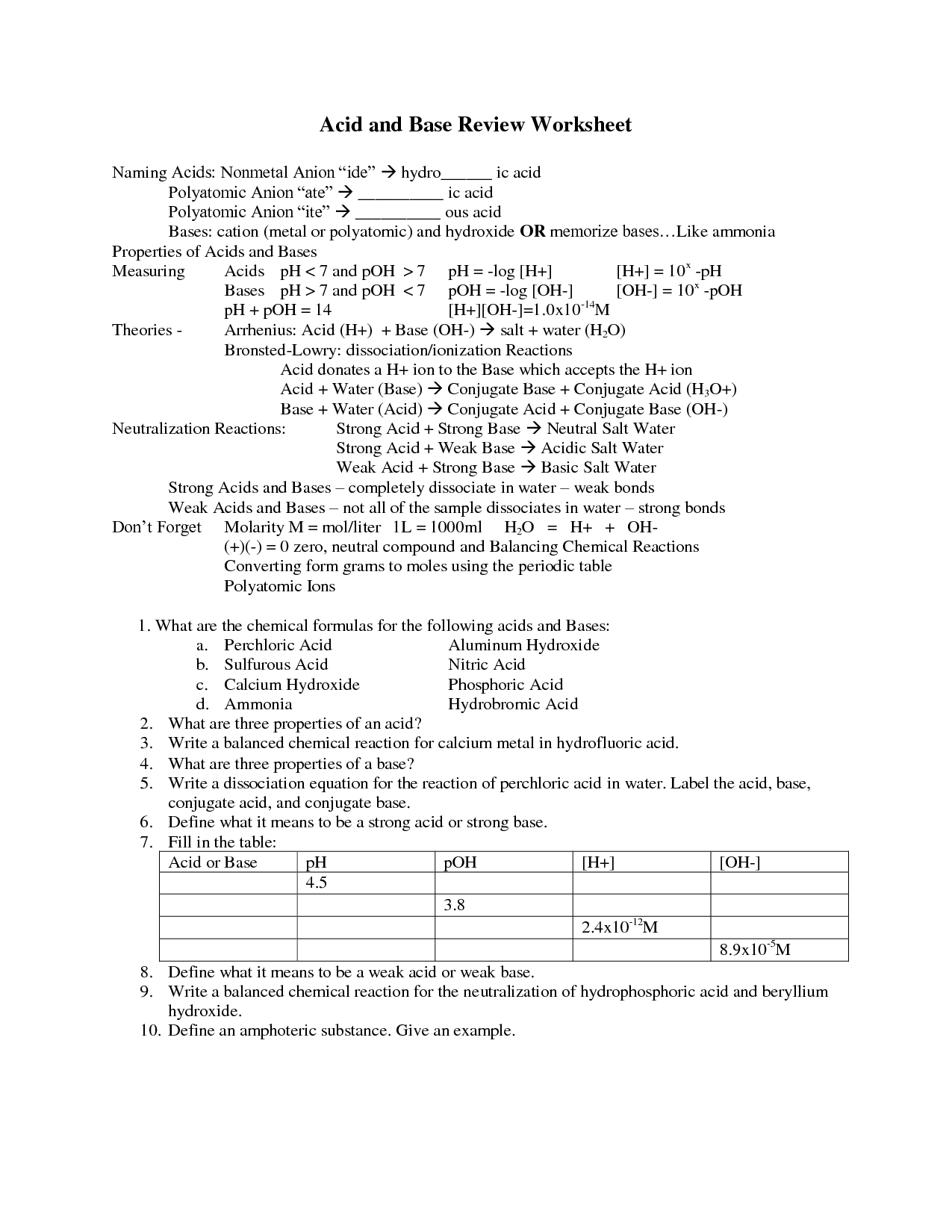
Acid nomenclature, also known as the systematic naming of acids, involves using specific rules and conventions to name various types of acids based on their structure and composition. For binary acids (composed of hydrogen and a nonmetal), the name includes the prefix "hydro-" followed by the nonmetal stem name with the suffix "-ic acid." For oxyacids (composed of hydrogen, oxygen, and a nonmetal), the name typically includes the nonmetal stem name followed by the term "ate" or "ite" depending on the number of oxygen atoms present, with the suffix "-ic acid" for acids derived from "-ate" and "-ous acid" for acids derived from "-ite.
What is the purpose of naming acids?
The purpose of naming acids is to categorize and identify them based on their chemical composition and properties. Naming conventions help in understanding the structure and behavior of acids, making it easier to communicate and study their characteristics, reactions, and uses in various fields such as chemistry, biology, and industry.
How do you name binary acids?
Binary acids are named by using the prefix "hydro-" followed by the root of the second element, with the suffix "-ic acid" added at the end. For example, HCl is named hydrochloric acid.
What is the difference between a binary acid and an oxyacid?
A binary acid is composed of just two elements - hydrogen and a non-metal, while an oxyacid contains hydrogen, oxygen, and another element. Binary acids do not contain oxygen, while oxyacids do. Additionally, oxyacids are typically named with the prefix "hydro-" and the suffix "-ic" or "-ous" depending on the oxidation state of the central non-metal element, whereas binary acids are named with the prefix "hydro-" and the suffix "-ic" followed by the word "acid.
How do you name oxyacids?
Oxyacids are named by combining the prefix "hydro-" with the root name of the nonmetal element, adding the suffix "-ic," followed by the word "acid." If the oxyacid has one more oxygen atom than the oxyacid ending in "-ic," it is named with the prefix "per-" before the root name of the nonmetal element. If the oxyacid has one less oxygen atom than the oxyacid ending in "-ic," it is named with the prefix "hypo-" before the root name of the nonmetal element, followed by the suffix "-ous" and the word "acid.
What is the role of the prefix "hydro-" in naming binary acids?
The prefix "hydro-" is used in naming binary acids to indicate that the acid is made up of hydrogen combined with one other nonmetallic element. This prefix is followed by the root name of the nonmetallic element, and the name of the acid ends with the suffix "-ic.
How do you determine the oxidation state of the central atom in oxyacids?
To determine the oxidation state of the central atom in oxyacids, you first need to know the oxidation states of the other elements in the compound. By considering the overall charge of the molecule and applying rules about how different elements form bonds and share electrons, you can calculate the oxidation state of the central atom. In oxyacids, the oxidation state of the oxygen atom is typically -2, so by subtracting the known oxidation states of the other elements from the overall charge, you can deduce the oxidation state of the central atom.
How do you name oxyacids containing a polyatomic ion?
To name oxyacids containing a polyatomic ion, you typically use the suffix "-ic" if the polyatomic ion ends in "-ate" and the suffix "-ous" if the polyatomic ion ends in "-ite." For example, H2SO4 is sulfuric acid (from sulfate ion SO4^2-) and HNO2 is nitrous acid (from nitrite ion NO2^-).
How does the number of oxygen atoms affect the naming of oxyacids?
The number of oxygen atoms in oxyacids affects their naming by changing the prefix used in the name. For oxyacids with fewer oxygen atoms, the prefix "hypo-" is used, whereas for oxyacids with the highest number of oxygen atoms, the prefix "per-" is used. The prefixes "ite" and "ate" are used for oxyacids with decreasing or increasing numbers of oxygen atoms in between.
What is the significance of acid nomenclature in chemistry?
Acid nomenclature in chemistry is significant as it helps to systematically name and classify different types of acids based on their composition and properties. By using a standardized naming system, chemists can effectively communicate information about acids, including their chemical structure, reactivity, and behavior in various reactions. This allows for clearer understanding, organization, and categorization of acids, facilitating research, education, and the practical application of acids in various fields of chemistry.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.




























Comments