6th Grade Chemistry Worksheets
If you're a 6th-grade student excited about delving into the captivating world of chemistry, then you've come to the right place. In this blog post, we will explore the benefits of using worksheets as a tool to enhance your understanding and application of fundamental chemistry concepts. Worksheets provide an organized and structured approach to engage with various chemical entities and subjects, making it easier for you to grasp key principles and reinforce your learning.
Table of Images 👆
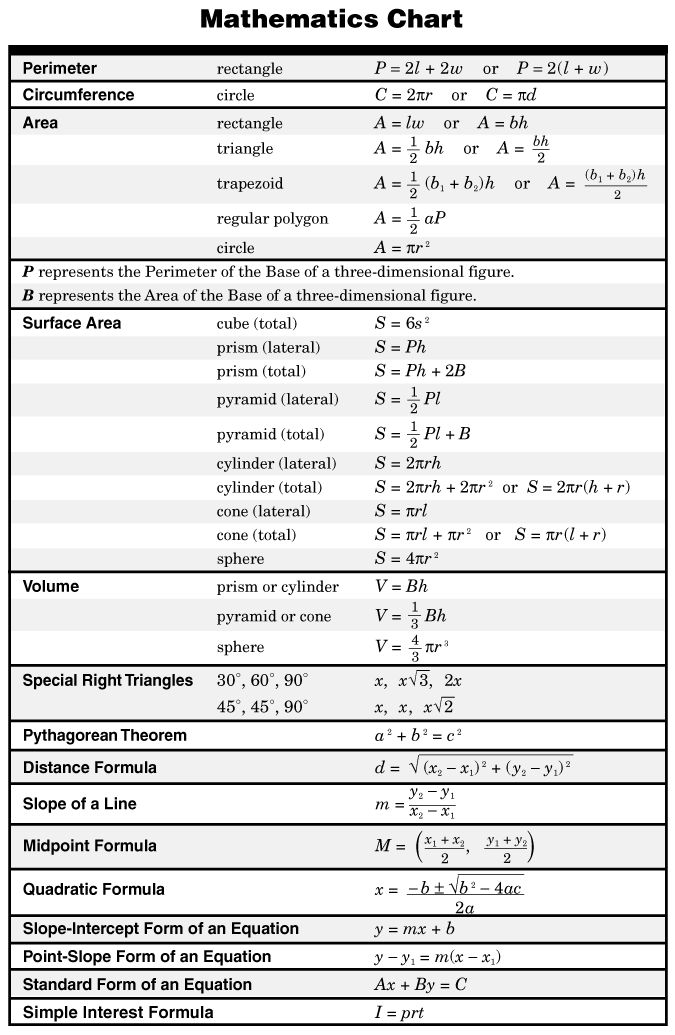
- Math for 7th Grade Formula Chart
- High School 9th Grade Biology Worksheets
- Chemistry Worksheets
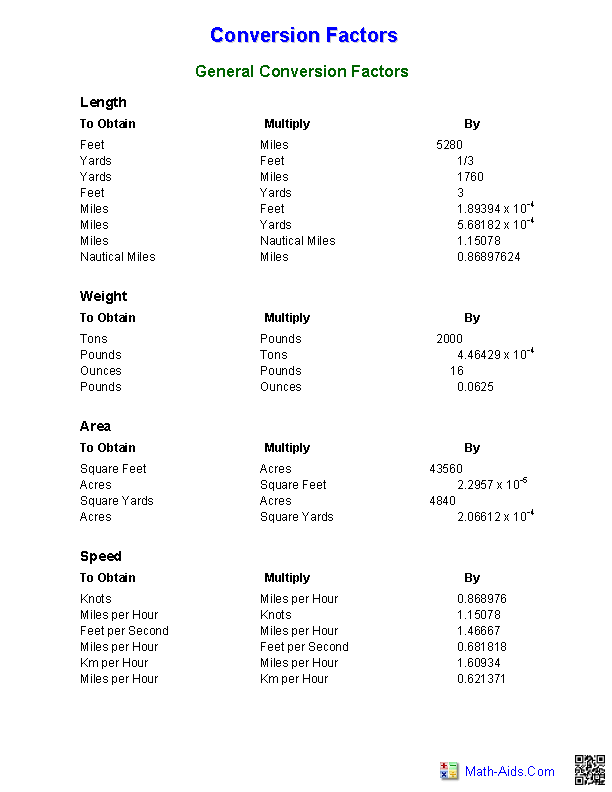
- Math Measurements Conversion Chart
- GCF and LCM Word Problems Worksheet Grade 6
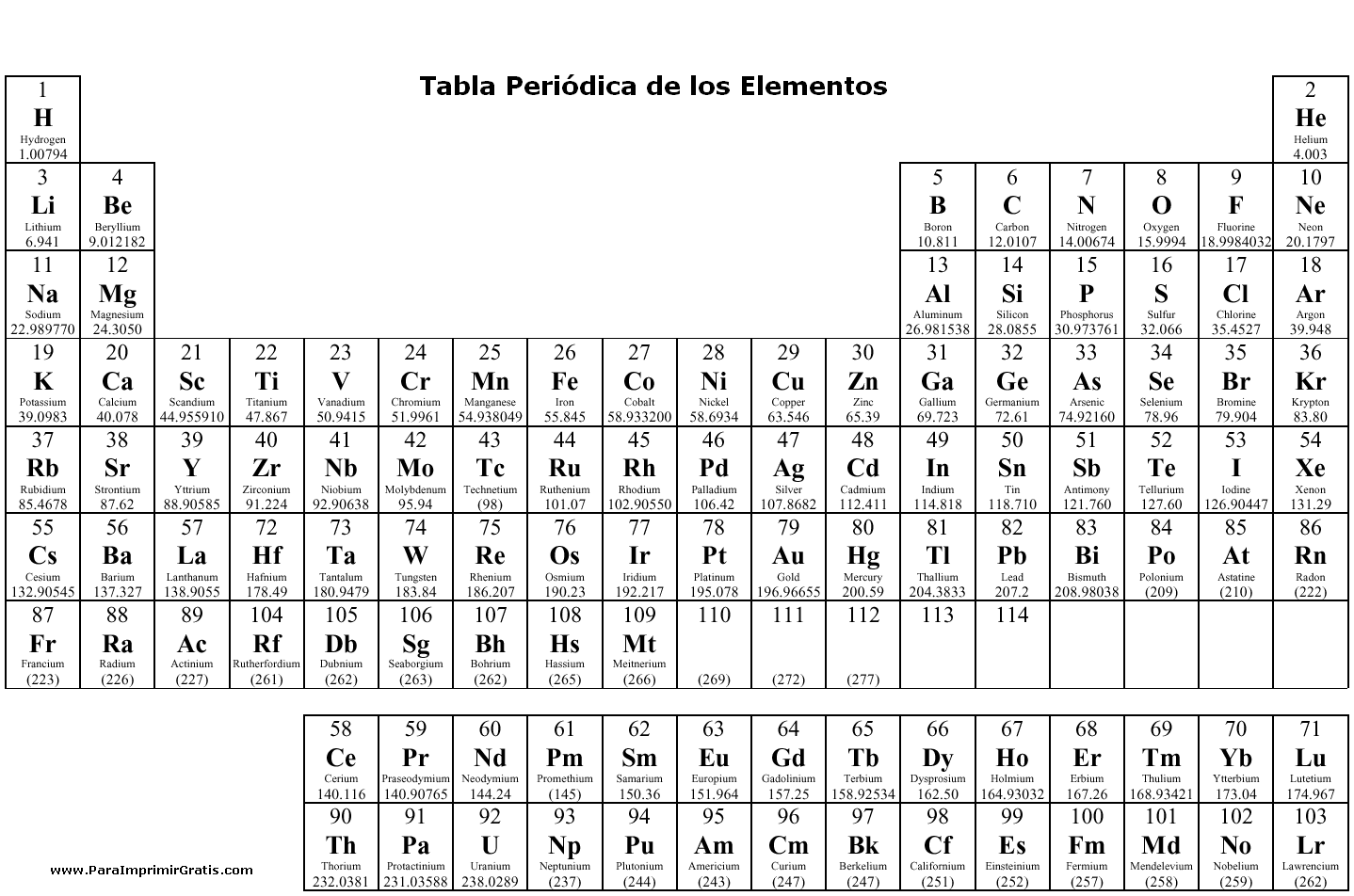
- Periodic Table with Element Charges
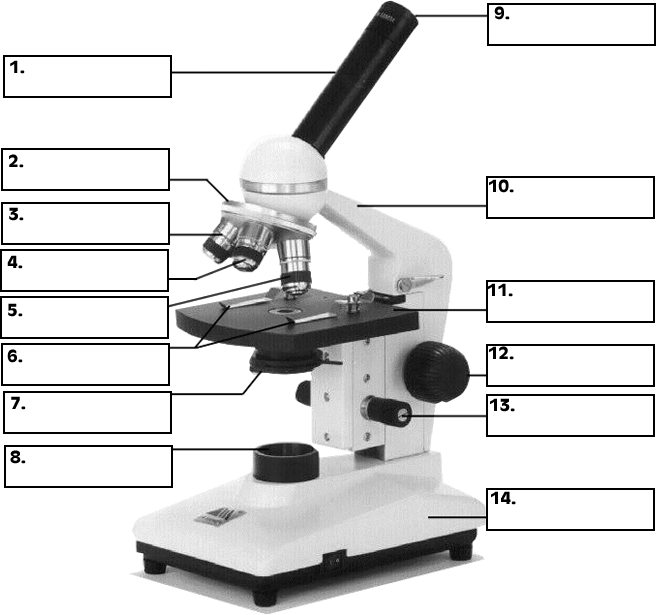
- Microscope Parts Quiz Worksheet
- Christmas Math Worksheets Printable
- 4th Grade Math Worksheet Packet
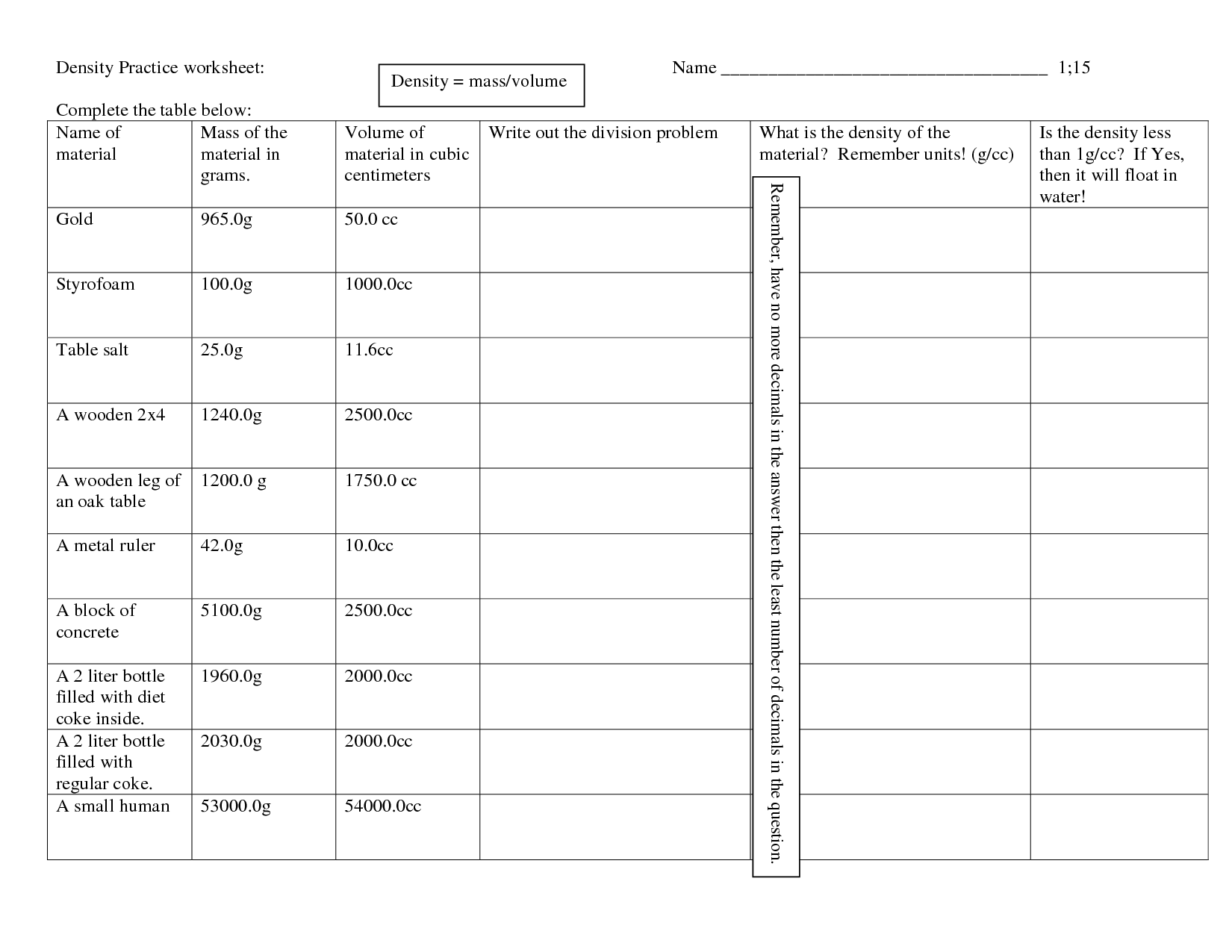
- Volume and Density Worksheet Middle School
More Chemistry Worksheets
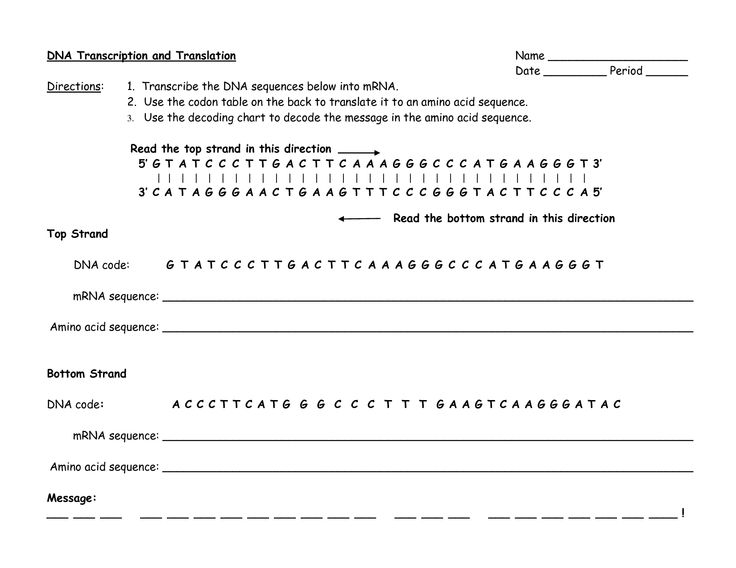
Chemistry Lab Equipment WorksheetChemistry Stoichiometry Worksheet Answer Key
Chemistry Conversion Factors Worksheet
Fun Chemistry Worksheets
What is the atomic number?
The atomic number of an element is the number of protons found in the nucleus of an atom. It determines the identity of an element and is used to organize elements in the periodic table.
Define an element.
In chemistry, an element is a substance that cannot be broken down into simpler substances by chemical means. Elements are made up of atoms that all have the same number of protons in their nucleus, giving them unique chemical properties. The periodic table organizes all known elements based on their atomic number and chemical properties.
What is an atom?
An atom is the basic unit of matter, consisting of a nucleus containing protons and neutrons with electrons orbiting around it. Atoms are the building blocks of all elements in the periodic table and combine to form molecules through chemical bonding.
Explain the difference between a compound and a mixture.
A compound is a substance made up of two or more different elements chemically bonded together in specific proportions, resulting in a unique substance with distinct properties, while a mixture is a combination of two or more substances that are physically mixed together but not chemically bonded, allowing each component to retain its own properties. Essentially, compounds have fixed compositions and properties, whereas mixtures can have variable compositions and properties.
Describe the structure of an atom.
An atom consists of a nucleus at its center, composed of protons and neutrons, surrounded by a cloud of electrons. Protons carry a positive charge, electrons carry a negative charge, and neutrons have no charge. The number of protons in the nucleus determines the element, while the number of electrons determines the atom's overall charge. Electrons occupy energy levels or shells around the nucleus, with the innermost shell holding the lowest energy electrons and subsequent shells holding higher energy electrons. The nucleus is extremely dense compared to the surrounding electron cloud, with the majority of an atom's mass concentrated in the nucleus.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
Define a chemical change.
A chemical change is a process in which one or more substances are transformed into new substances with different chemical compositions and properties, typically involving the breaking and forming of chemical bonds. This transformation is irreversible and results in the production of new substances that are distinct from the original substances.
Explain the concept of density.
Density is a physical property that measures the amount of mass in a given volume of a substance. It is calculated by dividing the mass of an object by its volume. Objects with high density have more mass in a smaller volume, while objects with low density have less mass in a larger volume. Density is important in various fields such as physics, chemistry, and engineering as it can help determine the composition, buoyancy, and behavior of materials.
Describe the pH scale and its significance in chemistry.
The pH scale is a measure used to specify the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating alkalinity. pH is crucial in chemistry as it determines the reactivity, solubility, and behavior of substances. It influences biological processes, industrial processes, environmental studies, and more. Maintaining the proper pH is essential for various applications, such as agriculture, water treatment, and pharmaceuticals, highlighting the significance of the pH scale in chemistry.
What is the difference between physical and chemical properties?
Physical properties are characteristics of a substance that can be observed or measured without changing the substance's composition, such as color, shape, and size. On the other hand, chemical properties describe the behavior of a substance in reactions with other substances, indicating how the substance can undergo chemical changes or interact with other substances.
Have something to share?
Who is Worksheeto?
At Worksheeto, we are committed to delivering an extensive and varied portfolio of superior quality worksheets, designed to address the educational demands of students, educators, and parents.



















Comments